Topic: Atom And The Ion
Atom And The Ion
What is the number of electrons in an Al3+ ion?
(1) 10
(2) 13
(3) 3
(4) 16
What occurs when a magnesium atom becomes a magnesium ion?
(1) Electrons are gained and the oxidation number increases.
(2) Electrons are gained and the oxidation number decreases.
(3) Electrons are lost and the oxidation number increases.
(4) Electrons are lost and the oxidation number decreases.
When an F atom becomes an F− ion, the F atom
(1) gains a proton
(2) loses a proton
(3) gains an electron
(4) loses an electron
An ion that consists of 7 protons, 6 neutrons, and 10 electrons has a net charge of
(1) 4−
(2) 3−
(3) 3+
(4) 4+
When lithium reacts with bromine to form the compound LiBr, each lithium atom
(1) gains one electron and becomes a negatively charged ion
(2) gains three electrons and becomes a negatively charged ion
(3) loses one electron and becomes a positively charged ion
(4) loses three electrons and becomes a positively charged ion
Which Lewis electron-dot diagram represents a fluoride ion?
(1) 
(2) 
(3) ![]()
(4) ![]()
Compared to a potassium atom, a potassium ion has
(1) a smaller radius
(2) a larger radius
(3) fewer protons
(4) more protons
The atomic radius and the ionic radius for some Group 1 and some Group 17 elements are given in the tables below.

Estimate the radius of a Br ion.
Allow 1 credit for an ionic radius value greater than 181 pm and less than 220. pm.
The atomic number and corresponding atomic radius of the Period 3 elements are shown in the data table below.

Explain, in terms of electrons, the change in radius when a sodium atom becomes a sodium ion.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The radius of a sodium ion is smaller because the sodium atom lost one electron.
• An Na+ ion is smaller because it has one fewer electron shell.
The Lewis electron-dot diagrams for three substances are shown below.

Identify the noble gas that has atoms with the same electron configuration as the positive ion represented in diagram 1, when both the atoms and the ion are in the ground state.
Allow 1 credit. Acceptable responses include, but are not limited to:
• argon
• Ar
The formulas and names of four chloride compounds are shown in the table below.
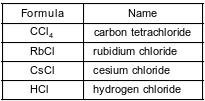
Explain, in terms of atomic structure, why the radius of a cesium ion in cesium chloride is smaller than the radius of a cesium atom when both are in the ground state.
Allow 1 credit. Acceptable responses include, but are not limited to:
• A cesium atom loses its valence electron, making the cesium ion smaller.
• The cesium atom has one more electron shell than the cesium ion.
• A Cs+ ion has only 5 shells of electrons in the ground state and the Cs atom has 6 shells.
John Dalton, an early scientist, sketched the structure of compounds using his own symbols for the elements known at the time. Dalton’s symbols for four elements and his drawing of potassium aluminum sulfate are represented by the diagram below.
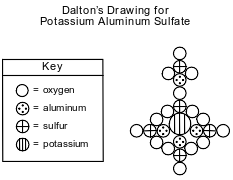
Today, it is known that the chemical formula for potassium aluminum sulfate is KAl(SO4)2\u202212H2O. It is a hydrated compound because water molecules are included within its crystal structure. There are 12 moles of H2O for every 1 mole of KAl(SO4)2. The compound contains two different positive ions. The gram-formula mass of KAl(SO4)2\u202212H2O is 474 grams per mole.
Identify one positive ion in the hydrated compound. Your response must include both the chemical symbol and charge of the ion.
Allow 1 credit. Acceptable responses include, but are not limited to:
• K+
• Al3+
When magnesium is ignited in air, the magnesium reacts with oxygen and nitrogen. The reaction between magnesium and nitrogen is represented by the unbalanced equation below.
Mg(s) + N2(g) → Mg3N2(s)
Explain, in terms of electrons, why an atom of the metal in this reaction forms an ion that has a smaller radius than its atom.
Allow 1 credit. Acceptable responses include, but are not limited to:
• An atom of magnesium loses its outer shell electrons to form the Mg2+ ion.
• The electron configuration of a magnesium atom is 2-8-2, and the electron configuration of the magnesium ion is 2-8.
• An atom of the metal loses electrons to form the ion.
Explain, in terms of electrons, why the radius of a potassium atom is larger than the radius of a potassium ion in the ground state.
Allow 1 credit. Acceptable responses include, but are not limited to:
• A potassium atom has four electron shells and a potassium ion has three electron shells.
• A potassium atom has one more electron shell than a potassium ion.
• A K+ ion has one fewer electron than a K atom.
A sample of seawater is analyzed. The table below gives the concentration of some ions in the sample.
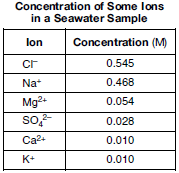
Compare the radius of an Mg2+ ion in the seawater to the radius of an Mg atom.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The radius of an Mg2+ ion is smaller than the radius of an Mg atom.
• The atom has a larger radius than the ion.
