Topic: Chemical Reactions And Equations
Chemical Reactions And Equations
Which quantities are conserved in all chemical reactions?
(1) charge, pressure, and energy
(2) charge, mass, and energy
(3) volume, pressure, and energy
(4) volume, mass, and pressure
Which quantities must be conserved in all chemical reactions?
(1) mass, charge, density
(2) mass, charge, energy
(3) charge, volume, density
(4) charge, volume, energy
What is conserved during all chemical reactions?
(1) charge
(2) density
(3) vapor pressure
(4) melting point
Given the balanced equation representing a reaction:
2Na(s) + Cl2(g) → 2NaCl(s) + energy
If 46 grams of Na and 71 grams of Cl2 react completely, what is the total mass of NaCl produced?
(1) 58.5 g
(2) 117 g
(3) 163 g
(4) 234 g
During all chemical reactions, charge, mass and energy are
(1) condensed
(2) conserved
(3) decayed
(4) decomposed
Which equation shows conservation of charge?
(1) Cu + Ag+ → Cu2+ + Ag
(2) Mg + Zn2+ → 2Mg2+ + Zn
(3) 2F2 + Br− → 2F− + Br2
(4) 2I− + Cl2 → I2 + 2Cl−
In 1828, Friedrich Wöhler produced urea when he heated a solution of ammonium cyanate. This reaction is represented by the balanced equation below.
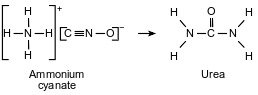
Explain why this balanced equation represents a conservation of atoms.
Allow 1 credit. Acceptable responses include, but are not limited to:
• There are the same number of atoms of each element on both sides of the equation.
• No atoms are lost or gained.
During a laboratory activity, appropriate safety equipment was used and safety procedures were followed. A laboratory technician heated a sample of solid KClO3 in a crucible to determine the percent composition by mass of oxygen in the compound. The unbalanced equation and the data for the decomposition of solid KClO3 are shown below.
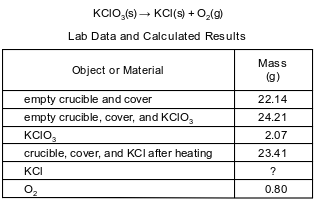
Based on the lab data, determine the mass of KCl produced in the reaction.
Allow 1 credit for 1.27 g.
• 69
• chem62019-rg_g3.png
Iron has been used for thousands of years. In the air, iron corrodes. One reaction for the corrosion of iron is represented by the balanced equation below.
Equation 1: 4Fe(s) + 3O2(g) → 2Fe2O3(s)
In the presence of water, iron corrodes more quickly. This corrosion is represented by the unbalanced equation below.
Equation 2: Fe(s) + O2(g) + H2O(ℓ) → Fe(OH)2(s)
Balance the equation in your answer booklet, using the smallest whole-number coefficients.
Allow 1 credit for __9__ Fe(s) + _______O2(g) + __9__ H2O(ℓ ) → __9__ Fe(OH)2(s).
• Allow credit even if the coefficient “1” is written in front of O2(g).
Baking soda, NaHCO3, can be commercially produced during a series of chemical reactions called the Solvay process. In this process, NH3(aq), NaCl(aq), and other chemicals are used to produce NaHCO3(s) and NH4Cl(aq).
To reduce production costs, NH3(aq) is recovered from NH4Cl(aq) through a different series of reactions. This series of reactions can be summarized by the overall reaction represented by the unbalanced equation below.
NH4Cl(aq) + CaO(s) → NH3(aq) + H2O(ℓ) + CaCl2(aq)
Balance the equation below for the overall reaction used to recover NH3(aq), using the smallest whole-number coefficients.

Allow 1 credit for 2 NH4Cl + CaO → 2 NH3 + H2O + CaCl2.
• Note: Allow credit even if the coefficient “1” is written in front of CaO, H2O, and/or CaCl2.
The balanced equation below represents the reaction of glucose, C6H12O6, with oxygen at 298 K and 101.3 kPa.
C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(ℓ)
Determine the mass of CO2 produced when 9.0 grams of glucose completely reacts with 9.6 grams of oxygen to produce 5.4 grams of water.
Allow 1 credit for 13.2 g or for any value from 13.155 g to 13.2042 g, inclusive.
The nuts, bolts, and hinges that attach some gates to a playground fence can be made of iron. The iron can react with oxygen in the air. The unbalanced equation representing this reaction is shown below.
Fe(s) + O2(g) → Fe2O3(s)
Balance the equation below for the reaction, using the smallest whole-number coefficients.

Allow 1 credit for 4___ Fe(s) + 3___ O2(g) → 2___ Fe2O3(s).
A NaOH(aq) solution and an acid-base indicator are used to determine the molarity of an HCl(aq) solution. A 25.0-milliliter sample of the HCl(aq) is exactly neutralized by 15.0 milliliters of 0.20 M NaOH(aq).
Using the data, determine the concentration of the HCl(aq).
Allow 1 credit for 0.12 M. Significant figures do not need to be shown.
During a titration, 10.00 mL of acetic acid, HC2H3O2(aq), is completely neutralized by adding 12.50 mL of 0.64 M sodium hydroxide, NaOH(aq).
Determine the molarity of the acetic acid.
Allow 1 credit. Acceptable responses include, but are not limited to:
• 0.80 M
• 8.0 × 10−1 M
• .8 M
Molecules containing two carbon atoms and a functional group have many home and industrial uses. These compounds can be produced by a variety of reactions, as shown by the equations below.
Equation 1: C2H4 + H2O → CH3CH2OH
Equation 2: 2CH3CH2OH + O2 → 2CH3CHO + 2H2O
Equation 3: 2CH3CHO + O2 → 2CH3COOH
Determine the number of moles of oxygen required to completely react with six moles of CH3CHO in equation 3.
Allow 1 credit for 3 mol or three mol.
