Topic: Compounds
Compounds
What is the IUPAC name for the compound ZnO?
(1) zinc oxide
(2) zinc oxalate
(3) zinc peroxide
(4) zinc hydroxide
Which sample of matter is classified as a substance?
(1) air
(2) ammonia
(3) milk
(4) seawater
In the formula XF2, the element represented by X can be classified as a
(1) Group 1 metal
(2) Group 2 metal
(3) Group 1 nonmetal
(4) Group 2 nonmetal
What is the chemical formula for zinc carbonate?
(1) ZnCO3
(2) Zn(CO3)2
(3) Zn2CO3
(4) Zn3CO2
Given the formulas of four organic compounds:

Which compounds have the same molecular formula?
(1) A and B
(2) A and C
(3) D and B
(4) D and C
Given the incomplete equation representing a reaction:
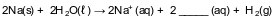
What is the formula of the missing product?
(1) O2−
(2) O2
(3) OH−
(4) OH
Which type of formula represents the simplest whole-number ratio of atoms of the elements in a compound?
(1) molecular formula
(2) condensed formula
(3) empirical formula
(4) structural formula
Two hydrocarbons that are isomers of each other are represented by the structural formulas and molecular formulas below.
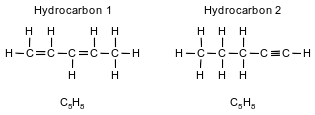
Explain, in terms of structural formulas and molecular formulas, why these hydrocarbons are isomers of each other.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The molecular formulas of the two hydrocarbons are the same, but the structural formulas are different.
During a fireworks display, salts are heated to very high temperatures. Ions in the salts absorb energy and become excited. Spectacular colors are produced as energy is emitted from the ions in the form of light.
The color of the emitted light is characteristic of the metal ion in each salt. For example, the lithium ion in lithium carbonate, Li2CO3, produces a deep-red color. The strontium ion in strontium carbonate, SrCO3, produces a bright-red color. Similarly, calcium chloride is used for orange light, sodium chloride for yellow light, and barium chloride for green light.
Write the formula for the salt used to produce green light in a fireworks display.
Allow 1 credit for BaCl2.
Vitamin C, also known as ascorbic acid, is water soluble and cannot be produced by the human body. Each day, a person’s diet should include a source of vitamin C, such as orange juice. Ascorbic acid has a molecular formula of C6H8O6 and a gram-formula mass of 176 grams per mole.
Determine the number of moles of vitamin C in an orange that contains 0.071 gram of vitamin C.
Allow 1 credit. Significant figures do not need to be shown. Acceptable responses include, but are
• not limited to:
• 4.0 × 10−4 mol
• 0.000 40 mol
The equation below represents the reaction between 1-butene and bromine to form the compound 1,2-dibromobutane, C4H8Br2.

Determine the gram-formula mass of 1-butene.
Allow 1 credit for 56 g/mol. Significant figures do not need to be shown.
Nitrogen gas and oxygen gas make up about 99% of Earth’s atmosphere. Other atmospheric gases include argon, carbon dioxide, methane, ozone, hydrogen, etc.
The amount of carbon dioxide in the atmosphere can vary. Data for the concentration of CO2(g) from 1960 to 2000 are shown in the table below.

Explain, in terms of types of matter, why methane can be broken down by chemical means, but argon can not be broken down by chemical means. Your response must include both methane and argon.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Methane is a compound consisting of two elements, so it can be broken down by chemical means, but argon is an element, which cannot be broken down.
• Methane is a compound and argon is an element.
The element boron, a trace element in Earth’s crust, is found in foods produced from plants. Boron has only two naturally occurring stable isotopes, boron-10 and boron-11.
One sample of a green vegetable contains 0.0035 gram of boron. Determine the total number of moles of boron in this sample.
Allow 1 credit for 0.000 32 mol or 3.2 × 10−4 mol. Significant figures do not need to be shown.
The active ingredient in the pain reliever aspirin is acetylsalicylic acid. This compound can be produced by reacting salicylic acid with acetic acid. The label of one aspirin bottle indicates that the accepted mass of acetylsalicylic acid in each tablet is 325 milligrams.
In a laboratory, an aspirin tablet is crushed and mixed with water to dissolve all of the acetylsalicylic acid. The measured pH of the resulting solution is 3.0.
Write the chemical formula for the acetic acid.
Allow 1 credit. Acceptable responses include, but are not limited to:
• HC2H3O2(aq)
• CH3COOH
Ethene and hydrogen can react at a faster rate in the presence of the catalyst platinum. The equation below represents a reaction between ethene and hydrogen.
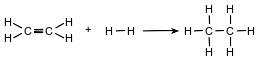
Determine the molar mass of the product.
Allow 1 credit for 30 g/mol, 30. g/mol, or for any value from 30.06 g/mol to 30.1 g/mol, inclusive.
