Topic: Concentration Of A Solution
Concentration Of A Solution
A 2400.-gram sample of an aqueous solution contains 0.012 gram of NH3. What is the concentration of NH3 in the solution, expressed as parts per million?
(1) 5.0 ppm
(2) 15 ppm
(3) 20. ppm
(4) 50. ppm
The concentration of a solution can be expressed in
(1) milliliters per minute
(2) parts per million
(3) grams per kelvin
(4) joules per gram
Parts per million is used to express the
(1) atomic mass of an element
(2) concentration of a solution
(3) volume of a substance
(4) rate of heat transfer
What is the molarity of a solution that contains 0.500 mole of KNO3 dissolved in 0.500-liter of solution?
(1) 1.00 M
(2) 2.00 M
(3) 0.500 M
(4) 4.00 M
A solution contains 25 grams of KNO3 dissolved in 200. grams of H2O. Which numerical setup can be used to calculate the percent by mass of KNO3 in this solution?
(1) 
(2) 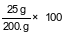
(3) 
(4) 
In a titration, a few drops of an indicator are added to a flask containing 35.0 milliliters of HNO3(aq) of unknown concentration. After 30.0 milliliters of 0.15 M NaOH(aq) solution is slowly added to the flask, the indicator changes color, showing the acid is neutralized.
Show a numerical setup for calculating the concentration of the HNO3(aq) solution.
Allow 1 credit. Acceptable responses include, but are not limited to:
• MA(35.0 mL) = (0.15 M)(30.0 mL)
• chem12012-rg_g3.png
Ammonium chloride is dissolved in water to form a 0.10 M NH4Cl(aq) solution. This dissolving process is represented by the equation below.

Determine the number of moles of NH4Cl(s) used to produce 2.0 liters of this solution.
Allow 1 credit for 0.20 mol. Significant figures do not need to be shown.
Nitrogen gas and oxygen gas make up about 99% of Earth’s atmosphere. Other atmospheric gases include argon, carbon dioxide, methane, ozone, hydrogen, etc.
The amount of carbon dioxide in the atmosphere can vary. Data for the concentration of CO2(g) from 1960 to 2000 are shown in the table below.

Show a numerical setup for calculating the mass of carbon dioxide in a 100.0-gram sample of air taken in 1980.
Allow 1 credit. Acceptable responses include, but are not limited to:
• 338.7 ppm =
• chem12013-rg_g3.png
In a titration, 50.0 milliliters of 0.026 M HCl(aq) is neutralized by 38.5 milliliters of KOH(aq).
Show a numerical setup for calculating the molarity of the KOH(aq).
Allow 1 credit. Acceptable responses include, but are not limited to:
• (0.026 M)(50.0 mL) = MB (38.5 mL)
• chem12014-rg_g1.png
Seawater contains dissolved salts in the form of ions. Some of the ions found in seawater are Ca2+, Mg2+, K+, Na+, Cl−, HCO3−, and SO42−. An investigation was conducted to determine the concentration of dissolved salts in seawater at one location. A 300.-gram sample of the seawater was placed in an open container. After a week, all the water had evaporated and 10. grams of solid salts remained in the container.
Determine the concentration, expressed as percent by mass, of the dissolved salts in the original sample of seawater.
Allow 1 credit. Acceptable responses include, but are not limited to:
• 3.3%
• 3%
• 3.3333%
Copper can be used for water pipes in homes. When the pipes corrode, copper atoms oxidize to form Cu2+ ions in the water.
A homeowner has a water quality report prepared for a sample of water taken from pipes in the home. According to the report, the 550.-gram sample contains 6.75 × 10−4 gram of dissolved Cu2+ ions.
Show a numerical setup for calculating the concentration, in parts per million, of dissolved Cu2+ ions in the sample of water tested.
Allow 1 credit. Acceptable responses include, but are not limited to:
• chem12015-rg_g8.png
A NaOH(aq) solution and an acid-base indicator are used to determine the molarity of an HCl(aq) solution. A 25.0-milliliter sample of the HCl(aq) is exactly neutralized by 15.0 milliliters of 0.20 M NaOH(aq).
Based on the data, the calculated molarity of the HCl(aq) solution should be expressed to what number of significant figures?
Allow 1 credit for 2 or two.
During a titration, 10.00 mL of acetic acid, HC2H3O2(aq), is completely neutralized by adding 12.50 mL of 0.64 M sodium hydroxide, NaOH(aq).
State the number of significant figures used to express the volume of the acetic acid.
Allow 1 credit for 4 or four.
A solution of ethylene glycol and water can be used as the coolant in an engine-cooling system. The ethylene glycol concentration in a coolant solution is often given as percent by volume. For example, 100. mL of a coolant solution that is 40.% ethylene glycol by volume contains 40. mL of ethylene glycol diluted with enough water to produce a total volume of 100. mL. The graph below shows the freezing point of coolants that have different ethylene glycol concentrations.
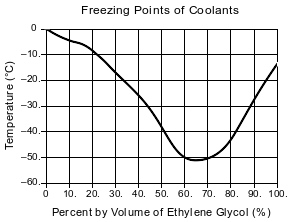
One engine-cooling system has a volume of 6400 mL. Determine the volume of ethylene glycol in the completely filled engine-cooling system when the concentration of ethylene glycol is 50.% by volume.
Allow 1 credit. Acceptable responses include, but are not limited to:
A student constructs an electrochemical cell. A diagram of the operating cell and the unbalanced ionic equation representing the reaction occurring in the cell are shown below.
The blue color of the solution in the copper half-cell indicates the presence of Cu2+ ions. The student observes that the blue color becomes less intense as the cell operates.
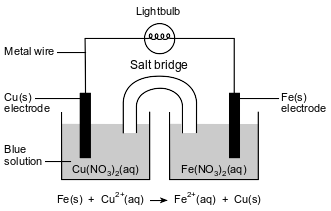
State one inference that the student can make about the concentration of the Cu2+ ions based on the change in intensity of the color of the Cu(NO3)2(aq) solution as the cell operates.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The concentration of the Cu2+ ions decreases.
• There are fewer copper ions in the solution.
