Topic: Electrolytic Cell
Electrolytic Cell
Which energy conversion must occur in an operating electrolytic cell?
(1) electrical energy to chemical energy
(2) electrical energy to nuclear energy
(3) chemical energy to electrical energy
(4) chemical energy to nuclear energy
An electrolytic cell differs from a voltaic cell because an electrolytic cell
(1) generates its own energy from a spontaneous physical reaction
(2) generates its own energy from a nonsponta- neous physical reaction
(3) requires an outside energy source for a spontaneous chemical reaction to occur
(4) requires an outside energy source for a nonspontaneous chemical reaction to occur
Which device requires electrical energy to produce a chemical change?
(1) electrolytic cell
(2) salt bridge
(3) voltaic cell
(4) voltmeter
A compound is broken down by chemical means during
(1) chromatography
(2) distillation
(3) electrolysis
(4) filtration
Energy is required to produce a chemical change during
(1) chromatography
(2) electrolysis
(3) boiling
(4) melting
Which process requires energy for a nonspontaneous redox reaction to occur?
(1) deposition
(2) electrolysis
(3) alpha decay
(4) chromatography
Which process requires energy to decompose a substance?
(1) electrolysis
(2) neutralization
(3) sublimation
(4) synthesis
Given the diagram representing an incomplete electrochemical cell:
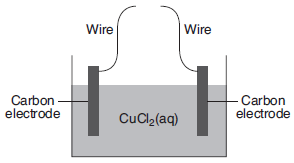
Solid copper will be deposited on one of the carbon electrodes when the wires are connected to
(1) each other
(2) a battery
(3) a switch
(4) a voltmeter
Which energy conversion occurs in an operating electrolytic cell?
(1) chemical energy to electrical energy
(2) electrical energy to chemical energy
(3) nuclear energy to electrical energy
(4) electrical energy to nuclear energy
The diagram below represents an operating electrolytic cell used to plate silver onto a nickel key. As the cell operates, oxidation occurs at the silver electrode and the mass of the silver electrode decreases.
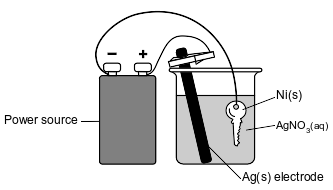
State the purpose of the power source in the cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The cell requires electrical energy for the nonspontaneous reaction to occur.
• The power source causes some Ag(s) atoms to oxidize.
Metallic elements are obtained from their ores by reduction. Some metals, such as zinc, lead, iron, and copper, can be obtained by heating their oxides with carbon.
More active metals, such as aluminum, magnesium, and sodium, can not be reduced by carbon. These metals can be obtained by the electrolysis of their molten (melted) ores. The diagram below represents an incomplete cell for the electrolysis of molten NaCl. The equation below represents the reaction that occurs when the completed cell operates.
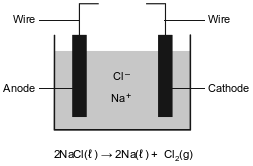
Identify the component required for the electrolysis of molten NaCl that is missing from the cell diagram.
Allow 1 credit. Acceptable responses include, but are not limited to:
• source of electrical energy
• battery
Fossil fuels produce air pollution and may eventually be depleted. Scientists are researching ways to use hydrogen as an alternate fuel.
A device called an artificial leaf was invented to produce hydrogen and oxygen using sunlight and water. The artifical leaf is an electrochemical cell. Equations 1 and 2 below represent the reactions taking place in the leaf. Equation 3 represents a reaction of hydrogen when used as fuel.
Equation 1: 2H2O + energy from sunlight → O2 + 4H+ + 4e−
Equation 2: 4H+ + 4e− → 2H2
Equation 3: 2H2(g) + O2(g) → 2H2O(g) + energy
Explain, in terms of energy, why the artificial leaf is an electrolytic cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Sunlight is used as an external power source for the cell.
• Sunlight is required to cause a nonspontaneous chemical change.
• Energy is required.
A student develops the list shown below that includes laboratory equipment and materials for constructing a voltaic cell.

Identify one item of laboratory equipment required to build an electrolytic cell that is not included in the list.
Allow 1 credit. Acceptable responses include, but are not limited to:
• battery
• external power source
• source of electricity
An operating voltaic cell has magnesium and silver electrodes. The cell and the ionic equation representing the reaction that occurs in the cell are shown below.
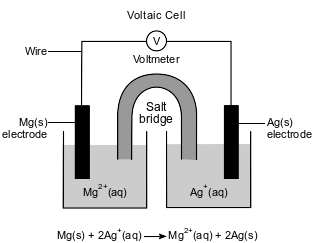
Explain, in terms of electrical energy, how electrolysis reactions differ from voltaic cell reactions.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Electrical energy is required for electrolytic reactions, while voltaic cell reactions produce electricity.
• Voltaic cells produce electrical energy, and electrolytic cells use electrical energy.
• Electrolysis reactions require an external source of electricity.
The electrolysis of brine, a concentrated aqueous sodium chloride solution, produces three important industrial chemicals: chlorine gas, hydrogen gas, and sodium hydroxide. The diagram and equation below represent a brine electrolysis cell. Before the battery is connected, the pH value of the brine solution is 7.0.

Explain, in terms of energy, why this cell is an electrolytic cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Electrical energy is required to cause a non-spontaneous chemical change.
• The battery supplies the electricity needed for the reaction to occur.
