Topic: Electron Dot Diagrams (Lewis Structures)
Electron Dot Diagrams (Lewis Structures)
Which Lewis electron-dot diagram represents a molecule having a nonpolar covalent bond?
(1) 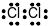
(2) 
(3) 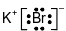
(4) 
Which Lewis electron-dot diagram represents a nitrogen atom in the ground state?
(1) ![]()
(2) 
(3) ![]()
(4) 
Which Lewis electron-dot diagram represents a fluoride ion?
(1) 
(2) 
(3) ![]()
(4) ![]()
Which Lewis electron-dot diagram represents the bonding in potassium iodide?
(1) 
(2) 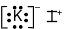
(3) ![]()
(4) ![]()
Which Lewis electron-dot diagram represents a molecule of H2S?
(1) ![]()
(2) ![]()
(3) 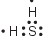
(4) 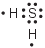
Which Lewis electron-dot diagram represents an atom of nitrogen in the ground state?
(1) ![]()
(2) ![]()
(3) ![]()
(4) ![]()
The diagrams below represent ball-and-stick models of two molecules. In a ball-and-stick model, each ball represents an atom, and the sticks between balls represent chemical bonds.

Draw a Lewis electron-dot diagram for an atom of the element present in all organic compounds.
Allow 1 credit. The positions of the dots can vary.
• Examples of 1-credit responses:
• chem12015-rg_g2.png
Draw a Lewis electron-dot diagram for an atom of silicon.
Allow 1 credit.
• Examples of 1-credit responses:
• chem12013-rg_g1.png
Silver-plated utensils were popular before stainless steel became widely used to make eating utensils. Silver tarnishes when it comes in contact with hydrogen sulfide, H2S, which is found in the air and in some foods. However, stainless steel does not tarnish when it comes in contact with hydrogen sulfide.
Draw a Lewis electron-dot diagram for the compound that tarnishes silver.
Allow 1 credit. The position of electrons may vary.
• Examples of 1-credit responses:
• chem12014-rg_g4.png
The Lewis electron-dot diagrams for three substances are shown below.

Describe, in terms of valence electrons, how the chemical bonds form in the substance represented in diagram 1.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Valence electrons are lost by potassium and gained by bromine.
• The ions form as a result of a transfer of electrons between the atoms.
Draw a Lewis electron-dot diagram for a molecule of hydrogen fluoride, HF.
Allow 1 credit.
• Examples of 1-credit responses:
• chem12018-rg_g1.png
The formulas and names of four chloride compounds are shown in the table below.
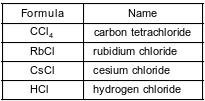
In the space in your answer booklet, draw a Lewis electron-dot diagram for a molecule of HCl.
Allow 1 credit.
• Examples of 1-credit responses:
• chem12019-rg_g1.png
The balanced equation below represents a reaction.
O2(g) + energy → O(g) + O(g)
In the space in your answer booklet, draw a Lewis electron-dot diagram of one oxygen atom.
Allow 1 credit.
• Examples of 1-credit responses:
• chem62013-rg_g1.png
Draw a Lewis electron-dot diagram for a molecule of bromomethane, CH3Br.
Allow 1 credit. The position of the electrons can vary.
• Examples of 1-credit responses:
• chem62014-rg_g1.png
Draw a Lewis electron-dot diagram for a chloride ion, Cl−.
Allow 1 credit. The position of electrons may vary.
• Examples of 1-credit responses:
• chem62016-rg_g1.png
