Topic: Elements In The Periodic Table
Elements In The Periodic Table
The elements on the Periodic Table are arranged in order of increasing
(1) atomic mass
(2) atomic number
(3) molar mass
(4) oxidation number
The elements on the Periodic Table are arranged in order of increasing
(1) mass number
(2) atomic number
(3) number of isotopes
(4) number of valence electrons
In which group on the Periodic Table would a nonmetallic element belong if atoms of this element tend to gain two electrons to complete their valence shell?
(1) 14
(2) 15
(3) 16
(4) 17
The elements on the Periodic Table of the Elements are arranged in order of increasing
(1) atomic number
(2) mass number
(3) number of neutrons
(4) number of valence electrons
Element X reacts with chlorine to form an ionic compound that has the formula XCl2. To which group on the Periodic Table could element X belong?
(1) Group 1
(2) Group 2
(3) Group 13
(4) Group 15
Which element has atoms that can form halide ions?
(1) iodine
(2) silver
(3) strontium
(4) xenon
What is the atomic number of the element whose atoms bond to each other in chains, rings, and networks?
(1) 10
(2) 8
(3) 6
(4) 4
In the formula for the compound XCl4, the X could represent
(1) C
(2) H
(3) Mg
(4) Zn
The elements in Period 4 on the Periodic Table are arranged in order of increasing
(1) atomic radius
(2) atomic number
(3) number of valence electrons
(4) number of occupied shells of electrons
Identify the element in Period 3 that is an unreactive gas at STP.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Ar
• argon
• element 18
Before atomic numbers were known, Mendeleev developed a classification system for the 63 elements known in 1872, using oxide formulas and atomic masses. He used an R in the oxide formulas to represent any element in each group. The atomic mass was listed in parentheses after the symbol of each element. A modified version of Mendeleev’s classification system is shown in the table below.
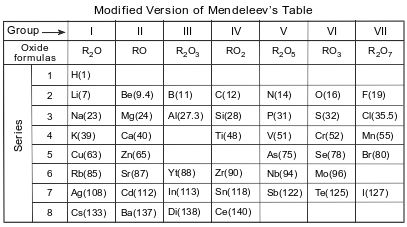
Identify one characteristic used by Mendeleev to develop his classification system of the elements.
Allow 1 credit. Acceptable responses include, but are not limited to:
• increasing atomic mass
• atomic mass
• oxide formulas
Three elements, represented by D, E, and Q, are located in Period 3. Some properties of these elements are listed in the table below. A student’s experimental result indicates that the density of element Q is 2.10 g/cm3, at room temperature and standard pressure.
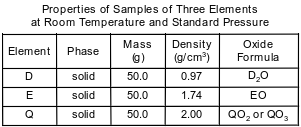
Identify the group on the Periodic Table to which element D belongs.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Group 1
• alkali metals
- A test tube contains a sample of solid stearic acid, an organic acid.
- Both the sample and the test tube have a temperature of 22.0°C.
- The stearic acid melts after the test tube is placed in a beaker with 320. grams of water at 98.0°C.
- The temperature of the liquid stearic acid and water in the beaker reaches 74.0°C.
Identify the element in stearic acid that makes it an organic compound.
Allow 1 credit for C or carbon.
In the late 1800s, Dmitri Mendeleev developed a periodic table of the elements known at that time. Based on the pattern in his periodic table, he was able to predict properties of some elements that had not yet been discovered. Information about two of these elements is shown in the table below.

Write a chemical formula for the compound formed between Ea and Cl.
Allow 1 credit. Acceptable responses include, but are not limited to:
• EaCl3
• GaCl3
Ethyl ethanoate is used as a solvent for varnishes and in the manufacture of artificial leather. The formula below represents a molecule of ethyl ethanoate.
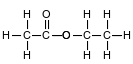
Identify the element in ethyl ethanoate that makes it an organic compound.
Allow 1 credit for C or carbon.
