Topic: Features Of Mixture
Features Of Mixture
Salt water is classified as a
(1) compound because the proportion of its atoms is fixed
(2) compound because the proportion of its atoms can vary
(3) mixture because the proportion of its components is fixed
(4) mixture because the proportion of its components can vary
Powdered iron is magnetic, but powdered sulfur is not. What occurs when they form a mixture in a beaker at room temperature?
(1) The iron retains its magnetic properties.
(2) The iron loses its metallic properties.
(3) The sulfur gains magnetic properties.
(4) The sulfur gains metallic properties.
Powdered sulfur is yellow, and powdered iron is gray. When powdered sulfur and powdered iron are mixed at 20°C, the powdered iron
(1) becomes yellow
(2) becomes a liquid
(3) remains ionic
(4) remains magnetic
Which property of an unsaturated solution of sodium chloride in water remains the same when more water is added to the solution?
(1) density of the solution
(2) boiling point of the solution
(3) mass of sodium chloride in the solution
(4) percent by mass of water in the solution
Distillation of crude oil from various parts of the world yields different percentages of hydrocarbons. Which statement explains these different percentages?
(1) Each component in a mixture has a different solubility in water.
(2) Hydrocarbons are organic compounds.
(3) The carbons in hydrocarbons may be bonded in chains or rings.
(4) The proportions of components in a mixture can vary.
Which statement describes the components of a mixture?
(1) Each component gains new properties.
(2) Each component loses its original properties.
(3) The proportions of components can vary.
(4) The proportions of components cannot vary.
The ratio of chromium to iron to carbon varies among the different types of stainless steel. Therefore, stainless steel is classified as
(1) a compound
(2) an element
(3) a mixture
(4) a substance
A 1.0-gram sample of NaCl(s) is dissolved in 100. grams of water at 25°C, and another 1.0-gram sample of NaCl(s) is dissolved in 50. grams of water at 25°C. Which property of the two resulting mixtures will be different?
(1) color of the components in the mixture
(2) particle size of the components in the mixture
(3) polarity of the components in the mixture
(4) proportion by mass of the components in the mixture
A sample of KCl(s) is dissolved in water to form KCl(aq). When the water in the KCl(aq) is completely evaporated, KCl(s) remains. Which statement describes a property of the KCl(s) after the water evaporated?
(1) The KCl(s) becomes a molecular compound.
(2) The molar mass of the KCl(s) decreases.
(3) The melting point of the KCl(s) is unchanged.
(4) The KCl(s) conducts an electric current.
A student prepares two 141-gram mixtures, A and B. Each mixture consists of NH4Cl, sand, and H2O at 15°C. Both mixtures are thoroughly stirred and allowed to stand. The mass of each component used to make the mixtures is listed in the data table below.
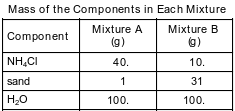
State evidence from the table indicating that the proportion of the components in a mixture can vary.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The ratio by mass of NH4Cl to H2O in mixture A is 40. g/100. g, and the ratio in mixture B is 10. g/100. g.
• Both mixtures have the same total mass, but have different amounts of sand.
• Mixture B has more sand.
• The mixtures have different proportions of NH4Cl.
Rubbing alcohol sold in stores is aqueous 2-propanol, CH3CHOHCH3(aq). Rubbing alcohol is available in concentrations of 70.% and 91% 2-propanol by volume.
To make 100. mL of 70.% aqueous 2-propanol, 70. mL of 2-propanol is diluted with enough water to produce a total volume of 100. mL. In a laboratory investigation, a student is given a 132-mL sample of 91% aqueous 2-propanol to separate using the process of distillation.
State evidence that indicates the proportions of the components in rubbing alcohol can vary.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The aqueous solutions of 2-propanol do not contain the same proportions of alcohol and water.
• Rubbing alcohol is sold as 70.% and 91% solutions.
During a laboratory activity, appropriate safety equipment is used and safety procedures are followed. A student separates a sample of rock salt that has two components; NaCl(s) and small insoluble rock particles. First, the student thoroughly stirs the sample of rock salt into a sample of water in a flask. The mixture in the flask is filtered using the lab apparatus shown below.

The water is evaporated from the beaker. The filter paper and its contents are dried. The data collected by the student are shown in the table below.

State evidence, other than mass, from the information given that the components of rock salt have different properties. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• The NaCl is soluble in water, and the rock particles are insoluble.
• The mixture can be separated by filtration.
