Topic: Formulas
Formulas
Given the formulas of four organic compounds:

Which compounds have the same molecular formula?
(1) A and B
(2) A and C
(3) D and B
(4) D and C
Given the incomplete equation representing a reaction:
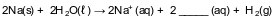
What is the formula of the missing product?
(1) O2−
(2) O2
(3) OH−
(4) OH
Which type of formula represents the simplest whole-number ratio of atoms of the elements in a compound?
(1) molecular formula
(2) condensed formula
(3) empirical formula
(4) structural formula
Which condensed structural formula represents an unsaturated compound?
(1) CH3CHCHCH3
(2) CH3CH2CH3
(3) CH3CH3
(4) CH4
Which formula is an empirical formula?
(1) CH4
(2) C2H6
(3) C3H6
(4) C4H10
Given the formula for a compound:

A chemical name for this compound is
(1) butanal
(2) butanol
(3) butanone
(4) butanoic acid
Which formula represents an alkane?
(1) C2H2
(2) C2H4
(3) C3H4
(4) C3H8
Two hydrocarbons that are isomers of each other are represented by the structural formulas and molecular formulas below.
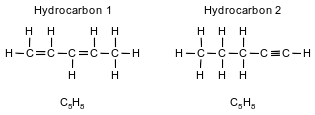
Explain, in terms of structural formulas and molecular formulas, why these hydrocarbons are isomers of each other.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The molecular formulas of the two hydrocarbons are the same, but the structural formulas are different.
During a fireworks display, salts are heated to very high temperatures. Ions in the salts absorb energy and become excited. Spectacular colors are produced as energy is emitted from the ions in the form of light.
The color of the emitted light is characteristic of the metal ion in each salt. For example, the lithium ion in lithium carbonate, Li2CO3, produces a deep-red color. The strontium ion in strontium carbonate, SrCO3, produces a bright-red color. Similarly, calcium chloride is used for orange light, sodium chloride for yellow light, and barium chloride for green light.
Write the formula for the salt used to produce green light in a fireworks display.
Allow 1 credit for BaCl2.
Vitamin C, also known as ascorbic acid, is water soluble and cannot be produced by the human body. Each day, a person’s diet should include a source of vitamin C, such as orange juice. Ascorbic acid has a molecular formula of C6H8O6 and a gram-formula mass of 176 grams per mole.
Write the empirical formula for ascorbic acid.
Allow 1 credit for C3H4O3. The order of the elements can vary.
The equation below represents the reaction between 1-butene and bromine to form the compound 1,2-dibromobutane, C4H8Br2.

Write the empirical formula for the product.
Allow 1 credit for C2H4Br. The order of the elements can vary.
The active ingredient in the pain reliever aspirin is acetylsalicylic acid. This compound can be produced by reacting salicylic acid with acetic acid. The label of one aspirin bottle indicates that the accepted mass of acetylsalicylic acid in each tablet is 325 milligrams.
In a laboratory, an aspirin tablet is crushed and mixed with water to dissolve all of the acetylsalicylic acid. The measured pH of the resulting solution is 3.0.
Write the chemical formula for the acetic acid.
Allow 1 credit. Acceptable responses include, but are not limited to:
• HC2H3O2(aq)
• CH3COOH
Rubbing alcohol is a product available at most pharmacies and supermarkets. One rubbing alcohol solution contains 2-propanol and water. The boiling point of 2-propanol is 82.3°C at standard pressure.
Draw a structural formula for the 2-propanol.
Allow 1 credit.
• Examples of 1-credit responses:
• chem12014-rg_g2.png
The balanced equation below represents the reaction of glucose, C6H12O6, with oxygen at 298 K and 101.3 kPa.
C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(ℓ)
Write the empirical formula for glucose.
Allow 1 credit for CH2O. The order of the elements can vary.
The diagrams below represent ball-and-stick models of two molecules. In a ball-and-stick model, each ball represents an atom, and the sticks between balls represent chemical bonds.

Explain why the molecules in diagrams A and B are isomers of each other.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Both molecules have the same molecular formula, but have different structural formulas.
• Both molecules are composed of 5 carbon atoms and 12 hydrogen atoms, but differ in the arrangement of their atoms.
