Topic: Half Reaction
Half Reaction
Given the balanced ionic equation representing a reaction:
2Al(s) + 3Cu2+(aq) → 2Al3+(aq) + 3Cu(s)
Which half-reaction represents the reduction that occurs?
(1) Al → Al3+ + 3e
(2) Al3+ + 3e → Al
(3) Cu → Cu2+ + 2e
(4) Cu2+ + 2e → Cu
Which half-reaction equation represents reduction?
(1) Cu → Cu2+ + 2e−
(2) Cu2+ + 2e− → Cu
(3) Ag + e− → Ag+
(4) Ag+ → Ag + e−
Which equation represents a reduction half- reaction?
(1) Fe → Fe3+ + 3e–
(2) Fe + 3e− → Fe3+
(3) Fe3+ → Fe + 3e–
(4) Fe3+ + 3e− → Fe
Which equation represents a reduction half-reaction?
(1) Na → Na+ + e−
(2) Na + e− → Na+
(3) Na+ → Na + e−
(4) Na+ + e− → Na
Which process can be represented by a halfreaction equation?
(1) distillation
(2) oxidation
(3) sublimation
(4) vaporization
Metallic elements are obtained from their ores by reduction. Some metals, such as zinc, lead, iron, and copper, can be obtained by heating their oxides with carbon.
More active metals, such as aluminum, magnesium, and sodium, can not be reduced by carbon. These metals can be obtained by the electrolysis of their molten (melted) ores. The diagram below represents an incomplete cell for the electrolysis of molten NaCl. The equation below represents the reaction that occurs when the completed cell operates.
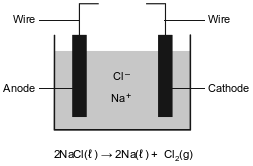
Write a balanced half-reaction equation for the reduction of the iron ions in iron(III) oxide to iron atoms.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Fe3+ + 3e− → Fe
In a laboratory apparatus, a sample of lead(II) oxide reacts with hydrogen gas at high temperature. The products of this reaction are liquid lead and water vapor. As the reaction proceeds, water vapor and excess hydrogen gas leave the glass tube. The diagram and balanced equation below represent this reaction.
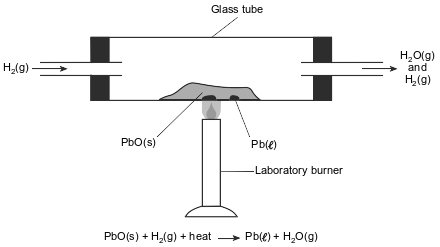
Write a balanced half-reaction equation for the reduction of the Pb2+ ions in this reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Pb2+ + 2e− → Pb
A student constructs an electrochemical cell during a laboratory investigation. When the switch is closed, electrons flow through the external circuit. The diagram and ionic equation below represent this cell and the reaction that occurs.

Write a balanced equation for the half-reaction that occurs in the Cu half-cell when the cell operates.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Cu2+(aq) + 2e− → Cu(s)
• 2e− + Cu+2 → Cu
Stamping an identification number into the steel frame of a bicycle compresses the crystal structure of the metal. If the number is filed off, there are scientific ways to reveal the number.
One method is to apply aqueous copper(II) chloride to the number area. The Cu2+ ions react with some iron atoms in the steel frame, producing copper atoms that show the pattern of the number. The ionic equation below represents this reaction.
Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
Another method is to apply hydrochloric acid to the number area. The acid reacts with the iron, producing bubbles of hydrogen gas. The bubbles form faster where the metal was compressed, so the number becomes visible. The equation below represents this reaction.
2HCl(aq) + Fe(s) → FeCl2(aq) + H2(g)
Write a balanced half-reaction equation for the reduction of the hydrogen ions to hydrogen gas.
Allow 1 credit. Acceptable responses include, but are not limited to:
• 2H+(aq) + 2e− → H2(g)
• 2H+ + 2e− → H2
• 2e− + 2H+1 → H2
The diagram and ionic equation below represent an operating voltaic cell.

Write a balanced equation for the half-reaction that occurs in the copper half-cell when the cell operates.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Cu2+(aq) + 2e− → Cu(s)
• Cu+2 + 2e− → Cu
• 3Cu2+ + 6e− → 3Cu
• Note: Do not allow credit for e without the (–) sign.
When solid copper is placed in an aqueous silver nitrate solution, a reaction occurs, as represented by the equation below.
Cu(s) + 2AgNO3(aq) → 2Ag(s) + Cu(NO3)2(aq)
Write a balanced half-reaction equation to represent the reduction of the silver ions to silver atoms. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• Ag+(aq) + e- → Ag(s)
• Ag+ + e- → Ag
• 2Ag+ + 2e- → 2Ag
• Note: Do not allow credit for the e without the minus sign (-).
During a laboratory activity appropriate safety equipment is used and safety procedures are followed. A student tests samples of four different metals using 0.20 M aqueous metal ion solutions of the same four metals. The student uses a 24-well plate as the reaction container for the different metal and solution combinations.
Before placing a metal strip in each solution, the student cleans the surface of the metal strip with sandpaper. The 24-well plate diagram below shows the setup and results of the investigation. In each vertical column, the metal strips are all the same metal. For each horizontal row, all of the solutions contain the same type of metal ion.
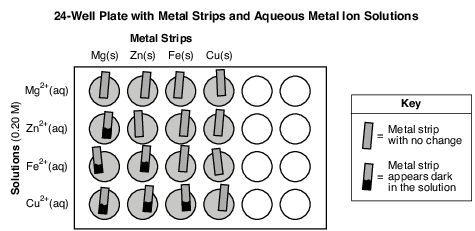
Write a balanced, half-reaction equation for the reduction of the copper ions. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• Cu2+(aq) + 2e− → Cu(s)
• Cu+2 + 2e− → Cu
• Note: Do not allow credit for the e without the minus sign (−).
During a laboratory activity, appropriate safety equipment is used and safety procedures are followed. A student constructs a voltaic cell with magnesium and copper electrodes. The diagram and net ionic equation below represent this cell and the reaction that occurs.
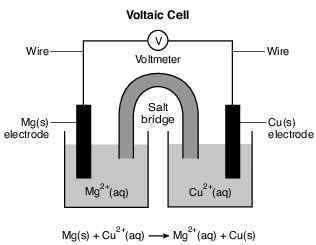
Write a balanced equation for the half-reaction that occurs in the copper half-cell when the cell operates. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• Cu2+(aq) + 2e− → Cu(s)
• Cu+2 + 2e− → Cu
• Note: Do not allow credit for the e without the minus sign (−).
Electroplating is an electrolytic process that can be used to coat metal objects with a less reactive metal. The diagram below shows an electroplating cell that includes a power source connected to a copper rod and a bracelet made from a different metal. The rod and bracelet are in an aqueous copper(II) sulfate solution.
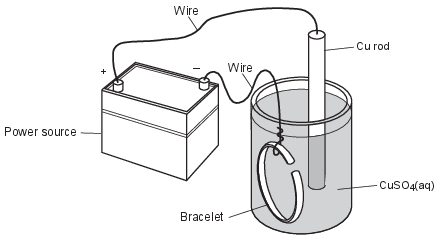
Write a balanced half-reaction equation for the reduction of Cu2+ ions that occurs in this cell. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• Cu2+(aq) + 2e- → Cu(s)
• Cu+2 + 2e- → Cu
• Note: Do not allow credit for the e without the minus sign (-).
Copper can be used for water pipes in homes. When the pipes corrode, copper atoms oxidize to form Cu2+ ions in the water.
A homeowner has a water quality report prepared for a sample of water taken from pipes in the home. According to the report, the 550.-gram sample contains 6.75 × 10−4 gram of dissolved Cu2+ ions.
Write a balanced half-reaction equation for the corrosion that forms the Cu2+ ions.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Cu → Cu2+ + 2e−
• Cu − 2e− → Cu+2
