Topic: Identification Of Element
Identification Of Element
The notation for the nuclide 13755Cs gives information about
(1) mass number, only
(2) atomic number, only
(3) both mass number and atomic number
(4) neither mass number nor atomic number
Which statement about one atom of an element identifies the element?
(1) The atom has 1 proton.
(2) The atom has 2 neutrons.
(3) The sum of the number of protons and neutrons in the atom is 3.
(4) The difference between the number of neutrons and protons in the atom is 1.
Which quantity represents the number of protons in an atom?
(1) atomic number
(2) oxidation number
(3) number of neutrons
(4) number of valence electrons
All phosphorus atoms have the same
(1) atomic number
(2) mass number
(3) number of neutrons plus the number of electrons
(4) number of neutrons plus the number of protons
The numbers of protons and neutrons in each of four different atoms are shown in the table below.
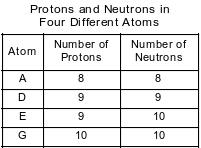
Which two atoms represent isotopes of the same element?
(1) A and D
(2) A and G
(3) E and D
(4) E and G
The nuclides I-131 and I-133 are classified as
(1) isomers of the same element
(2) isomers of Xe-131 and Cs-133
(3) isotopes of the same element
(4) isotopes of Xe-131 and Cs-133
What is the charge of the nucleus of a copper atom?
(1) +1
(2) +2
(3) +29
(4) +64
The three nuclides, U-233, U-235, and U-238, are isotopes of uranium because they have the same number of protons per atom and
(1) the same number of electrons per atom
(2) the same number of neutrons per atom
(3) a different number of electrons per atom
(4) a different number of neutrons per atom
What is the charge of the nucleus of an oxygen atom?
(1) 0
(2) −2
(3) +8
(4) +16
The element boron, a trace element in Earth’s crust, is found in foods produced from plants. Boron has only two naturally occurring stable isotopes, boron-10 and boron-11.
Write an isotopic notation of the heavier isotope of the element boron. Your response must include the atomic number, the mass number, and the symbol of this isotope.
Allow 1 credit for 115B.
Iodine has many isotopes, but only iodine-127 is stable and is found in nature. One radioactive iodine isotope, I-108, decays by alpha particle emission. Iodine-131 is also radioactive and has many important medical uses.
Determine the number of neutrons in an atom of I-127.
Allow 1 credit for 74.
A breeder reactor is one type of nuclear reactor. In a breeder reactor, uranium-238 is transformed in a series of nuclear reactions into plutonium-239.
The plutonium-239 can undergo fission as shown in the equation below. The X represents a missing product in the equation.

Determine the number of neutrons in an atom of the uranium isotope used in the breeder reactor.
Allow 1 credit for 146.
Elements with an atomic number greater than 92 can be artificially produced in nuclear reactions by bombarding a naturally occurring nuclide with a different nuclide. One of these elements is roentgenium, Rg. The equation below represents a nuclear reaction that produces Rg-272.

State the location and the total charge of the protons in a Ni-64 atom.
Allow 1 credit for a correct combination of the location and the total charge of the protons.
• Acceptable responses include, but are not limited to:
• Location of protons:
• in the nucleus
• the small, dense center of an atom
• center of an atom
• Total charge of protons:
• +28
• 28+
• 28
The radioisotope Mo-99 naturally decays to produce the metastable isotope Tc-99m, which is used in medical diagnosis. A doctor can obtain images of organs and bones by injecting a patient with a solution of Tc-99m. The half-life of the metastable Tc-99m is six hours.
State both the number of protons and the number of neutrons in a Tc-99 nuclide.
Allow 1 credit for 43 protons and 56 neutrons.
The diagram below shows the first three steps in the uranium-238 radioactive decay series.
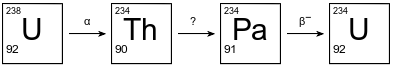
The decay mode for the first and third steps is shown above the arrows. The decay mode for the second step is not shown in the diagram. Thorium-234 has a half-life of 24.10 days.
Explain, in terms of neutrons and protons, why U-238 and U-234 are different isotopes of uranium.
Allow 1 credit. Acceptable responses include, but are not limited to:
• An atom of U-238 has 92 protons and 146 neutrons. An atom of U-234 also has 92 protons but has 142 neutrons.
• These two atoms have the same number of protons but a different number of neutrons.
