Topic: Nuclear Energy
Nuclear Energy
Given the balanced equation representing a nuclear reaction:
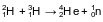
Which phrase identifies and describes this reaction?
(1) fission, mass converted to energy
(2) fission, energy converted to mass
(3) fusion, mass converted to energy
(4) fusion, energy converted to mass
Given the equation representing a reaction where the masses are expressed in atomic mass units:
hydrogen-2 + hydrogen-1 → helium-3 + 8.814 × 10−16 kJ
2.014 102 u 1.007 825 u 3.016 029 u
Which phrase describes this reaction?
(1) a chemical reaction and mass being converted to energy
(2) a chemical reaction and energy being converted to mass
(3) a nuclear reaction and mass being converted to energy
(4) a nuclear reaction and energy being converted to mass
During a nuclear reaction, mass is converted into
(1) charge
(2) energy
(3) isomers
(4) volume
Which change occurs during a nuclear fission reaction?
(1) Covalent bonds are converted to ionic bonds.
(2) Isotopes are converted to isomers.
(3) Temperature is converted to mass.
(4) Matter is converted to energy.
The energy released during a nuclear reaction is a result of
(1) breaking chemical bonds
(2) forming chemical bonds
(3) mass being converted to energy
(4) energy being converted to mass
Which net change occurs in a nuclear fusion reaction?
(1) Ionic bonds are broken.
(2) Ionic bonds are formed.
(3) Energy is converted to mass.
(4) Mass is converted to energy.
Which process converts mass into energy?
(1) distillation of ethanol
(2) filtration of a mixture
(3) fusion of hydrogen atoms
(4) ionization of cesium atoms
The energy released by a nuclear fusion reaction is produced when
(1) energy is converted to mass
(2) mass is converted to energy
(3) heat is converted to temperature
(4) temperature is converted to heat
Given the equation representing a reaction:
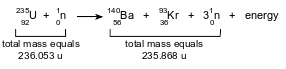
Which statement explains the energy term in this reaction?
(1) Mass is gained due to the conversion of mass to energy.
(2) Mass is gained due to the conversion of energy to mass.
(3) Mass is lost due to the conversion of mass to energy.
(4) Mass is lost due to the conversion of energy to mass.
Given the nuclear equation and isotopic masses:
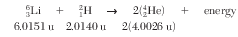
What is the amount of mass converted to energy as a result of the reaction between the two reactant nuclei?
(1) 0.0239 u
(2) 4.0265 u
(3) 8.0052 u
(4) 16.0343 u
Which net change occurs in both nuclear fission and nuclear fusion reactions?
(1) Mass is converted to energy.
(2) Energy is converted to mass.
(3) Small nuclei form a larger nucleus.
(4) A large nucleus forms smaller nuclei.
Which statement describes the net change that occurs during nuclear fission?
(1) Electrons are converted to protons.
(2) Protons are converted to electrons.
(3) Mass is converted to energy.
(4) Energy is converted to mass.
Which reaction releases the greatest amount of energy per kilogram of reactants?
(1) 10n + 23592U → 14156Ba +9236Kr + 310n
(2) 2C + H2 → C2H2
(3) C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(ℓ)
(4) NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(ℓ)
Nuclear fission is currently used to produce electricity in a nuclear power plant. One possible fission reaction is represented by the equation below.
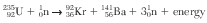
The barium-141 decays by beta emission and has a half-life of 18.3 minutes.
Compare the amount of energy released by the fission of one mole of uranium-235 to the amount of energy released by the combustion of one mole of octane fuel, C8H18. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• The fission of one mole of U-235 releases much more energy than the combustion of one mole of C8H18.
• For equal quantities, fission gives out more energy than combustion.
• Burning 1 mol of C8H18 releases much less energy.
• less energy from chemical reaction
A breeder reactor is one type of nuclear reactor. In a breeder reactor, uranium-238 is transformed in a series of nuclear reactions into plutonium-239.
The plutonium-239 can undergo fission as shown in the equation below. The X represents a missing product in the equation.

Compare the amount of energy released by 1 mole of completely fissioned plutonium-239 to the amount of energy released by the complete combustion of 1 mole of methane.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The fission of one mole of Pu-239 releases much more energy than the combustion of one mole of CH4.
• The energy released during the chemical reaction is less than the energy released during the nuclear reaction.
• greater for 23994Pu
