Topic: Number Of Paritcles And Valumes Of Gases
Number Of Paritcles And Valumes Of Gases
A sealed, rigid 1.0-liter cylinder contains He gas at STP. An identical sealed cylinder contains Ne gas at STP. These two cylinders contain the same number of
(1) atoms
(2) electrons
(3) ions
(4) protons
At STP, a 1-liter sample of Ne(g) and a 1-liter sample of Kr(g) have the same
(1) mass
(2) density
(3) number of atoms
(4) number of electrons
Which sample of gas at STP has the same number of molecules as 6 liters of Cl2(g) at STP?
(1) 3 liters of O2(g)
(2) 6 liters of N2(g)
(3) 3 moles of O2(g)
(4) 6 moles of N2(g)
At STP, which gas sample has the same number of molecules as 2.0 liters of CH4(g) at STP?
(1) 1.0 liter of C2H6(g)
(2) 2.0 liters of O2(g)
(3) 5.0 liters of N2(g)
(4) 6.0 liters of CO2(g)
Which gas sample at STP has the same number of molecules as a 2.0-liter sample of Cl2(g) at STP?
(1) 1.0 L of NH3(g)
(2) 2.0 L of CH4(g)
(3) 3.0 L of CO2(g)
(4) 4.0 L of NO(g)
At STP, which sample contains the same number of molecules as 3.0 liters of H2(g)?
(1) 1.5 L of NH3(g)
(2) 2.0 L of CO2(g)
(3) 3.0 L of CH4(g)
(4) 6.0 L of N2(g)
At STP, which gaseous sample has the same number of molecules as 3.0 liters of N2(g)?
(1) 6.0 L of F2(g)
(2) 4.5 L of N2(g)
(3) 3.0 L of H2(g)
(4) 1.5 L of Cl2(g)
The table below shows data for the temperature, pressure, and volume of four gas samples.
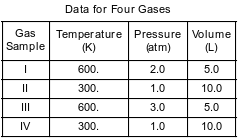
Which two gas samples contain the same number of molecules?
(1) I and II
(2) I and III
(3) II and III
(4) II and IV
At STP, a 12.0-liter sample of CH4(g) has the same total number of molecules as
(1) 6.0 L of H2(g) at STP
(2) 12.0 L of CO2(g) at STP
(3) 18.0 L of HCl(g) at STP
(4) 24.0 L of O2(g) at STP
Which sample at STP has the same number of atoms as 18 liters of Ne(g) at STP?
(1) 18 moles of Ar(g)
(2) 18 liters of Ar(g)
(3) 18 grams of H2O(g)
(4) 18 milliliters of H2O(g)
The volumes of four samples of gaseous compounds at 298 K and 101.3 kPa are shown in the table below.

Which two samples contain the same number of molecules?
(1) 1 and 2
(2) 1 and 3
(3) 2 and 3
(4) 2 and 4
Convert the melting point of mercury to degrees Celsius.
Allow 1 credit for −39°C.
Cylinder A has a movable piston and contains hydrogen gas. An identical cylinder, B, contains methane gas. The diagram below represents these cylinders and the conditions of pressure, volume, and temperature of the gas in each cylinder.
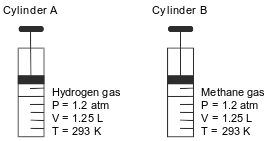
Compare the total number of gas molecules in cylinder A to the total number of gas molecules in cylinder B.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The number of gas molecules in cylinder A is the same as the number of gas molecules in cylinder B.
At 23°C, 85.0 grams of NaNO3(s) are dissolved in 100. grams of H2O(ℓ).
Convert the temperature of the NaNO3(s) to kelvins.
Allow 1 credit for 296 K.
Some properties of the element sodium are listed below.
- is a soft, silver-colored metal
- melts at a temperature of 371 K
- oxidizes easily in the presence of air
- forms compounds with nonmetallic elements in nature
- forms sodium chloride in the presence of chlorine gas
Convert the melting point of sodium to degrees Celsius.
Allow 1 credit for 98°C.
