Topic: Organic Compounds
Organic Compounds
Which element has atoms that can bond to each other in rings and networks?
(1) aluminum
(2) carbon
(3) hydrogen
(4) oxygen
Given the formulas of four organic compounds:

Which compounds have the same molecular formula?
(1) A and B
(2) A and C
(3) D and B
(4) D and C
Which formula represents an organic compound?
(1) CaH2
(2) C4H8
(3) H2O2
(4) P2O5
Which condensed structural formula represents an unsaturated compound?
(1) CH3CHCHCH3
(2) CH3CH2CH3
(3) CH3CH3
(4) CH4
Which element is present in all organic compounds?
(1) nitrogen
(2) oxygen
(3) carbon
(4) sulfur
Given the formula for a compound:

A chemical name for this compound is
(1) butanal
(2) butanol
(3) butanone
(4) butanoic acid
Which formula represents an alkane?
(1) C2H2
(2) C2H4
(3) C3H4
(4) C3H8
Given the formula representing a compound:

What is the IUPAC name of this compound?
(1) 2-chloroheptane
(2) 6-chloroheptane
(3) 2,2-dichloroheptane
(4) 6,6-dichloroheptane
Which formula represents an alkyne?
(1) CnHn
(2) C2nHn
(3) CnH2n + 2
(4) CnH2n– 2
Which compounds are isomers of each other?
(1) methanol and methanal
(2) propanoic acid and pentanoic acid
(3) 1-propanol and 2-propanol
(4) 1-chloropropane and 2-bromopropane
Two hydrocarbons that are isomers of each other are represented by the structural formulas and molecular formulas below.
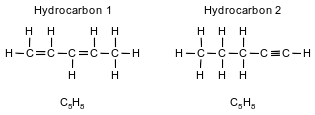
Explain, in terms of structural formulas and molecular formulas, why these hydrocarbons are isomers of each other.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The molecular formulas of the two hydrocarbons are the same, but the structural formulas are different.
The active ingredient in the pain reliever aspirin is acetylsalicylic acid. This compound can be produced by reacting salicylic acid with acetic acid. The label of one aspirin bottle indicates that the accepted mass of acetylsalicylic acid in each tablet is 325 milligrams.
In a laboratory, an aspirin tablet is crushed and mixed with water to dissolve all of the acetylsalicylic acid. The measured pH of the resulting solution is 3.0.
Write the chemical formula for the acetic acid.
Allow 1 credit. Acceptable responses include, but are not limited to:
• HC2H3O2(aq)
• CH3COOH
Table sugar, sucrose, is a combination of two simple sugars, glucose and fructose. The formulas below represent these simple sugars.

Explain, in terms of atoms and molecular structure, why glucose and fructose are isomers of each other.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The number of each kind of atom is the same in both, but their structures are not the same.
• Their molecular formulas are the same, but their structural arrangement of atoms is different.
• same molecular formula but different structural formulas
• The only difference is the arrangement of the atoms.
Rubbing alcohol is a product available at most pharmacies and supermarkets. One rubbing alcohol solution contains 2-propanol and water. The boiling point of 2-propanol is 82.3°C at standard pressure.
Draw a structural formula for the 2-propanol.
Allow 1 credit.
• Examples of 1-credit responses:
• chem12014-rg_g2.png
The diagrams below represent ball-and-stick models of two molecules. In a ball-and-stick model, each ball represents an atom, and the sticks between balls represent chemical bonds.

Explain why the molecules in diagrams A and B are isomers of each other.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Both molecules have the same molecular formula, but have different structural formulas.
• Both molecules are composed of 5 carbon atoms and 12 hydrogen atoms, but differ in the arrangement of their atoms.
