Topic: Oxidation Numbers (States)
Oxidation Numbers (States)
What is the oxidation number of iodine in KIO4?
(1) +1
(2) −1
(3) +7
(4) −7
What are the two oxidation states of nitrogen in NH4NO2?
(1) +3 and +5
(2) +3 and −5
(3) −3 and +3
(4) −3 and −3
What is the oxidation number of manganese in KMnO4?
(1) +7
(2) +2
(3) +3
(4) +4
What is the oxidation state for a Mn atom?
(1) 0
(2) +7
(3) +3
(4) +4
What occurs when a magnesium atom becomes a magnesium ion?
(1) Electrons are gained and the oxidation number increases.
(2) Electrons are gained and the oxidation number decreases.
(3) Electrons are lost and the oxidation number increases.
(4) Electrons are lost and the oxidation number decreases.
During a fireworks display, salts are heated to very high temperatures. Ions in the salts absorb energy and become excited. Spectacular colors are produced as energy is emitted from the ions in the form of light.
The color of the emitted light is characteristic of the metal ion in each salt. For example, the lithium ion in lithium carbonate, Li2CO3, produces a deep-red color. The strontium ion in strontium carbonate, SrCO3, produces a bright-red color. Similarly, calcium chloride is used for orange light, sodium chloride for yellow light, and barium chloride for green light.
Determine the oxidation state of carbon in the salt used to produce a bright-red color.
Allow 1 credit for +4.
Common household bleach is an aqueous solution containing hypochlorite ions. A closed container of bleach is an equilibrium system represented by the equation below.
Cl2(g) + 2OH−(aq) ⇌ ClO−(aq) + Cl−(aq) + H2O(ℓ)
State the change in oxidation number for chlorine when the Cl2(g) changes to Cl−(aq) during the forward reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
The nuts, bolts, and hinges that attach some gates to a playground fence can be made of iron. The iron can react with oxygen in the air. The unbalanced equation representing this reaction is shown below.
Fe(s) + O2(g) → Fe2O3(s)
Determine the change in oxidation state for oxygen in this reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
• From 0 to −2
• From 0 to 2−
• From zero to negative two
Fossil fuels produce air pollution and may eventually be depleted. Scientists are researching ways to use hydrogen as an alternate fuel.
A device called an artificial leaf was invented to produce hydrogen and oxygen using sunlight and water. The artifical leaf is an electrochemical cell. Equations 1 and 2 below represent the reactions taking place in the leaf. Equation 3 represents a reaction of hydrogen when used as fuel.
Equation 1: 2H2O + energy from sunlight → O2 + 4H+ + 4e−
Equation 2: 4H+ + 4e− → 2H2
Equation 3: 2H2(g) + O2(g) → 2H2O(g) + energy
State the change in oxidation number of oxygen during the reaction represented in equation 3.
Allow 1 credit. Acceptable responses include, but are not limited to:
• From 0 to −2
• From 0 to 2−
• From zero to negative two
The diagram and balanced ionic equation below represent two half-cells connected to produce an operating voltaic cell in a laboratory investigation. The half-cells are connected by a salt bridge.

Determine the oxidation number of nitrogen in the negative ion in the aqueous solutions.
Allow 1 credit for +5 or 5 or five.
Nitrogen dioxide, NO2, is a dark brown gas that is used to make nitric acid and to bleach flour. Nitrogen dioxide has a boiling point of 294 K at 101.3 kPa. In a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, N2O4. This equilibrium is represented by the equation below.
2NO2(g) ⇌ N2O4(g) + 58 kJ
Determine the oxidation state of nitrogen in nitrogen dioxide.
Allow 1 credit for +4 or 4+ or four.
In a laboratory apparatus, a sample of lead(II) oxide reacts with hydrogen gas at high temperature. The products of this reaction are liquid lead and water vapor. As the reaction proceeds, water vapor and excess hydrogen gas leave the glass tube. The diagram and balanced equation below represent this reaction.
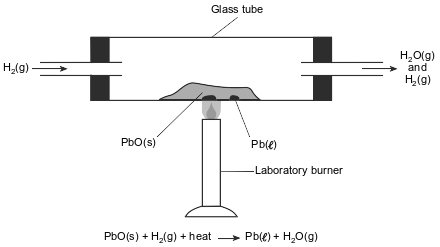
Determine the change in oxidation number for the hydrogen that reacts.
Allow 1 credit. Acceptable responses include, but are not limited to:
• From 0 to +1
• From zero to one
Fuel cells are voltaic cells. In one type of fuel cell, oxygen gas, O2(g), reacts with hydrogen gas, H2(g), producing water vapor, H2O(g), and electrical energy. The unbalanced equation for this redox reaction is shown below.
H2(g) + O2(g) → H2O(g) + energy
A diagram of the fuel cell is shown below. During operation of the fuel cell, hydrogen gas is pumped into one compartment and oxygen gas is pumped into the other compartment. Each compartment has an inner wall that is a porous carbon electrode through which ions flow. Aqueous potassium hydroxide, KOH(aq), and the porous electrodes serve as the salt bridge.

Determine the change in oxidation number for oxygen in this operating fuel cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• From 0 to −2
• From 0 to 2−
• From zero to negative 2
Early scientists defined oxidation as a chemical reaction in which oxygen combined with another element to produce an oxide of the element. An example of oxidation based on this definition is the combustion of methane. This reaction is represented by the balanced equation below.
Equation 1: CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
The definition of oxidation has since been expanded to include many reactions that do not involve oxygen. An example of oxidation based on this expanded definition is the reaction between magnesium ribbon and powdered sulfur when heated in a crucible. This reaction is represented by the balanced equation below.
Equation 2: Mg(s) + S(s) → MgS(s)
Determine the change in oxidation number of carbon in equation 1.
Allow 1 credit. Acceptable responses include, but are not limited to:
• from −4 to +4
• from negative four to four
One type of voltaic cell, called a mercury battery, uses zinc and mercury(II) oxide to generate an electric current. Mercury batteries were used because of their miniature size, even though mercury is toxic. The overall reaction for a mercury battery is given in the equation below.
Zn(s) + HgO(s) → ZnO(s) + Hg(ℓ)
Determine the change in the oxidation number of zinc during the operation of the cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• From 0 to +2
• From 0 to 2+
• From zero to two
