Topic: Oxidation Reduction (Redox) Reaction
Oxidation Reduction (Redox) Reaction
When a voltaic cell operates, ions move through the
(1) anode
(2) cathode
(3) salt bridge
(4) external circuit
Based on Reference Table J, which two reactants react spontaneously?
(1) Mg(s) + ZnCl2(aq)
(2) Cu(s) + FeSO4(aq)
(3) Pb(s) + ZnCl2(aq)
(4) Co(s) + NaCl(aq)
In an oxidation-reduction reaction, the total number of electrons lost is
(1) equal to the total number of electrons gained
(2) equal to the total number of protons gained
(3) less than the total number of electrons gained
(4) less than the total number of protons gained
Which metal will spontaneously react with Zn2+(aq), but will not spontaneously react with Mg2+(aq)?
(1) Mn(s)
(2) Cu(s)
(3) Ni(s)
(4) Ba(s)
Given the incomplete equation representing a reaction:
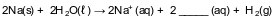
What is the formula of the missing product?
(1) O2−
(2) O2
(3) OH−
(4) OH
In an oxidation-reduction reaction, the number of electrons lost is
(1) equal to the number of electrons gained
(2) equal to the number of protons gained
(3) less than the number of electrons gained
(4) less than the number of protons gained
Which element reacts spontaneously with 1.0 M HCl(aq) at room temperature?
(1) copper
(2) gold
(3) silver
(4) zinc
Given the balanced ionic equation:
3Pb2+(aq) + 2Cr(s) → 3Pb(s) + 2Cr3+(aq)
What is the number of moles of electrons gained by 3.0 moles of lead ions?
(1) 5.0 mol
(2) 2.0 mol
(3) 3.0 mol
(4) 6.0 mol
Given the balanced equation representing a reaction:
2Al(s) + 3Cu2+(aq) → 2Al3+(aq) + 3Cu(s)
Which particles are transferred in this reaction?
(1) electrons
(2) neutrons
(3) positrons
(4) protons
The diagram below represents an operating electrolytic cell used to plate silver onto a nickel key. As the cell operates, oxidation occurs at the silver electrode and the mass of the silver electrode decreases.
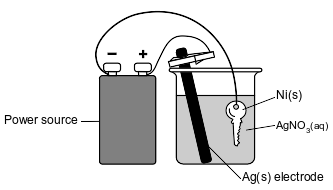
Explain, in terms of Ag atoms and Ag+(aq) ions, why the mass of the silver electrode decreases as the cell operates.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Silver atoms lose electrons and become silver ions in the solution.
• Some of the Ag atoms become Ag+ ions.
• Silver atoms are oxidized to silver ions.
Metallic elements are obtained from their ores by reduction. Some metals, such as zinc, lead, iron, and copper, can be obtained by heating their oxides with carbon.
More active metals, such as aluminum, magnesium, and sodium, can not be reduced by carbon. These metals can be obtained by the electrolysis of their molten (melted) ores. The diagram below represents an incomplete cell for the electrolysis of molten NaCl. The equation below represents the reaction that occurs when the completed cell operates.
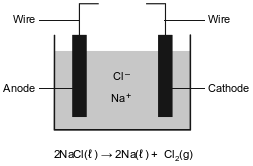
Identify one metal from the passage that is more active than carbon and one metal from the passage that is less active than carbon.
Allow 1 credit for identifying one metal from the passage that is more active than carbon and
• one metal from the passage that is less active than carbon.
• More active than carbon:
• aluminum
• Mg
• Na
• Less active than carbon:
• zinc
• Pb
• Fe
• copper
Fossil fuels produce air pollution and may eventually be depleted. Scientists are researching ways to use hydrogen as an alternate fuel.
A device called an artificial leaf was invented to produce hydrogen and oxygen using sunlight and water. The artifical leaf is an electrochemical cell. Equations 1 and 2 below represent the reactions taking place in the leaf. Equation 3 represents a reaction of hydrogen when used as fuel.
Equation 1: 2H2O + energy from sunlight → O2 + 4H+ + 4e−
Equation 2: 4H+ + 4e− → 2H2
Equation 3: 2H2(g) + O2(g) → 2H2O(g) + energy
State one benefit of using the artificial leaf to produce hydrogen.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The hydrogen could replace the use of fossil fuel.
• The use of hydrogen as a car fuel could reduce air pollution.
• The H2 fuel is renewable.
• Water is a nonpolluting product.
• The leaf uses renewable resources.
The diagram and balanced ionic equation below represent two half-cells connected to produce an operating voltaic cell in a laboratory investigation. The half-cells are connected by a salt bridge.

State the purpose of the salt bridge in this voltaic cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The salt bridge allows ions to flow between the two half-cells.
• It maintains the electrical neutrality of the solutions.
• prevents polarization of the half-cells
A student sets up a voltaic cell using magnesium and zinc electrodes. The porous barrier in the cell has the same purpose as a salt bridge. The diagram and the ionic equation below represent this operating cell.

State, in terms of ions, how the porous barrier functions as a salt bridge in this cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The porous barrier allows for the migration of ions between the half-cells.
• The barrier maintains electrical neutrality by allowing ions to flow.
A student constructs an electrochemical cell during a laboratory investigation. When the switch is closed, electrons flow through the external circuit. The diagram and equation below represent this cell and the reaction that occurs.

State the direction of electron flow through the wire when the switch is closed.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Electrons flow from the Al electrode to the Ni electrode.
• Electrons move left to right through the wire.
