Topic: Percent Composition
Percent Composition
Which compound has the smallest percent composition by mass of chlorine?
(1) HCl
(2) KCl
(3) LiCl
(4) NaCl
Which compound has the greatest percent composition by mass of sulfur?
(1) BaS
(2) CaS
(3) MgS
(4) SrS
Which compound has the highest percent com- position by mass of strontium?
(1) SrCl2
(2) SrI2
(3) SrO
(4) SrS
Which quantity can be calculated for a solid compound, given only the formula of the compound and the Periodic Table of the Elements?
(1) the density of the compound
(2) the heat of fusion of the compound
(3) the melting point of each element in the compound
(4) the percent composition by mass of each element in the compound
Vitamin C, also known as ascorbic acid, is water soluble and cannot be produced by the human body. Each day, a person’s diet should include a source of vitamin C, such as orange juice. Ascorbic acid has a molecular formula of C6H8O6 and a gram-formula mass of 176 grams per mole.
Show a numerical setup for calculating the percent composition by mass of oxygen in ascorbic acid.
Allow 1 credit. Acceptable responses include, but are not limited to:
• chem12012-rg_g4.png
Baking soda, NaHCO3, can be commercially produced during a series of chemical reactions called the Solvay process. In this process, NH3(aq), NaCl(aq), and other chemicals are used to produce NaHCO3(s) and NH4Cl(aq).
To reduce production costs, NH3(aq) is recovered from NH4Cl(aq) through a different series of reactions. This series of reactions can be summarized by the overall reaction represented by the unbalanced equation below.
NH4Cl(aq) + CaO(s) → NH3(aq) + H2O(ℓ) + CaCl2(aq)
Determine the percent composition by mass of carbon in baking soda (gram-formula mass = 84 grams per mole).
Allow 1 credit for 14% or for any value from 14.28% to 14.3%, inclusive.
Show a numerical setup for calculating the percent composition by mass of silicon in SiO2.
Allow 1 credit. Acceptable responses include, but are not limited to:
• chem12016-rg_g1.png
A sample of calcium carbonate, CaCO3, has a mass of 42.2 grams. Calcium carbonate has a gram-formula mass of 100. g/mol.
Determine the percent composition by mass of oxygen in the CaCO3.
Allow 1 credit for 48.0% or for any value from 47.9% to 48%, inclusive.
Given the unbalanced equation showing the reactants and product of a reaction occurring at 298 K and 100. kPa:
P4(s) + Cl2(g) → PCl3(ℓ) + energy
Show a numerical setup for calculating the percent composition by mass of chlorine in PCl3(ℓ) (gram-formula mass = 137 g/mol).
Allow 1 credit. Acceptable responses include, but are not limited to:
• chem12018-rg_g2.png
John Dalton, an early scientist, sketched the structure of compounds using his own symbols for the elements known at the time. Dalton’s symbols for four elements and his drawing of potassium aluminum sulfate are represented by the diagram below.
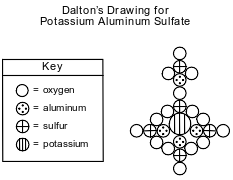
Today, it is known that the chemical formula for potassium aluminum sulfate is KAl(SO4)2\u202212H2O. It is a hydrated compound because water molecules are included within its crystal structure. There are 12 moles of H2O for every 1 mole of KAl(SO4)2. The compound contains two different positive ions. The gram-formula mass of KAl(SO4)2\u202212H2O is 474 grams per mole.
Show a numerical setup for calculating the percent composition by mass of water in KAl(SO4)2•12H2O.
Allow 1 credit. Acceptable responses include, but are not limited to:
• 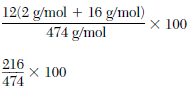
Some compounds of silver are listed with their chemical formulas in the table below.
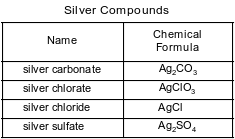
Show a numerical setup for calculating the percent composition by mass of silver in silver carbonate (gram-formula mass = 276 g/mol).
Allow 1 credit. Acceptable responses include, but are not limited to:
• chem62018-rg_g2.png
The two naturally occurring isotopes of antimony are Sb-121 and Sb-123. The table below shows the atomic mass and percent natural abundance for these isotopes.
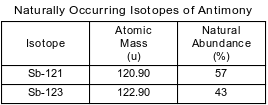
Antimony and sulfur are both found in the mineral stibnite, Sb2S3. To obtain antimony, stibnite is roasted (heated in air), producing oxides of antimony and sulfur. The unbalanced equation below represents one of the reactions that occurs during the roasting.
Sb2S3(s) + O2(g) → Sb2O3(s) + SO2(g)
Determine the percent composition by mass of antimony in stibnite (gram-formula mass = 340. g/mol).
Allow 1 credit for 71.6% or any value from 71.55% to 72%, inclusive.
Wood is mainly cellulose, a polymer produced by plants. One use of wood is as a fuel in campfires, fireplaces, and wood furnaces. The molecules of cellulose are long chains of repeating units. Each unit of the chain can be represented as C6H10O5. The balanced equation below represents a reaction that occurs when C6H10O5 is burned in air.
C6H10O5 + 6O2 → 6CO2 + 5H2O + heat
Show a numerical setup for calculating the percent composition by mass of carbon in C6H10O5 (gram-formula mass = 162.1 g/mol).
Allow 1 credit. Acceptable responses include, but are not limited to:
• chem82017-rg_g2.png
Show a numerical setup for calculating the percent composition by mass of oxygen in Al2O3 (gram-formula mass = 102 g/mol).
Allow 1 credit. Acceptable responses include, but are not limited to:
• chem82018-rg_g1.png
A hydrate is a compound that has water molecules within its crystal structure. Magnesium sulfate heptahydrate, MgSO4•7H2O, is a hydrated form of magnesium sulfate. The hydrated compound has 7 moles of H2O for each mole of MgSO4. When 5.06 grams of MgSO4•7H2O are heated to at least 300.°C in a crucible by using a laboratory burner, the water molecules are released. The sample was heated repeatedly, until the remaining MgSO4 had a constant mass of 2.47 grams. During this laboratory activity, appropriate safety equipment was used and safety procedures were followed.
Using the lab data, show a numerical setup for calculating the percent composition by mass of water in the hydrated compound.
Allow 1 credit. Acceptable responses include, but are not limited to:
• chem82019-rg_g2.png
