Topic: Periodic Table Of The Elements
Periodic Table Of The Elements
Which phrase describes an atom?
(1) a negatively charged nucleus surrounded by positively charged protons
(2) a negatively charged nucleus surrounded by positively charged electrons
(3) a positively charged nucleus surrounded by negatively charged protons
(4) a positively charged nucleus surrounded by negatively charged electrons
Which element has chemical properties that are most similar to the chemical properties of fluorine?
(1) boron
(2) chlorine
(3) neon
(4) oxygen
Which statement about one atom of an element identifies the element?
(1) The atom has 1 proton.
(2) The atom has 2 neutrons.
(3) The sum of the number of protons and neutrons in the atom is 3.
(4) The difference between the number of neutrons and protons in the atom is 1.
A solid element that is malleable, a good conductor of electricity, and reacts with oxygen is classified as a
(1) metal
(2) metalloid
(3) noble gas
(4) nonmetal
Which element has atoms that can bond to each other in rings and networks?
(1) aluminum
(2) carbon
(3) hydrogen
(4) oxygen
Which electron configuration represents a selenium atom in an excited state?
(1) 2-7-18-6
(2) 2-7-18-7
(3) 2-8-18-6
(4) 2-8-18-7
In the formula XF2, the element represented by X can be classified as a
(1) Group 1 metal
(2) Group 2 metal
(3) Group 1 nonmetal
(4) Group 2 nonmetal
What is the oxidation number of iodine in KIO4?
(1) +1
(2) −1
(3) +7
(4) −7
What is the chemical formula for zinc carbonate?
(1) ZnCO3
(2) Zn(CO3)2
(3) Zn2CO3
(4) Zn3CO2
The atomic number and corresponding atomic radius of the Period 3 elements are shown in the data table below.

State the general relationship between the atomic number and the atomic radius for the Period 3 elements.
Allow 1 credit. Acceptable responses include, but are not limited to:
• As atomic number increases, there is a decrease in atomic radius.
The equation below represents the reaction between 1-butene and bromine to form the compound 1,2-dibromobutane, C4H8Br2.

Determine the gram-formula mass of 1-butene.
Allow 1 credit for 56 g/mol. Significant figures do not need to be shown.
Metallic elements are obtained from their ores by reduction. Some metals, such as zinc, lead, iron, and copper, can be obtained by heating their oxides with carbon.
More active metals, such as aluminum, magnesium, and sodium, can not be reduced by carbon. These metals can be obtained by the electrolysis of their molten (melted) ores. The diagram below represents an incomplete cell for the electrolysis of molten NaCl. The equation below represents the reaction that occurs when the completed cell operates.
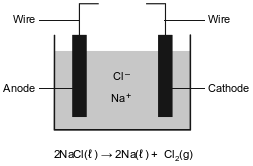
Write a balanced half-reaction equation for the reduction of the iron ions in iron(III) oxide to iron atoms.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Fe3+ + 3e− → Fe
The element boron, a trace element in Earth’s crust, is found in foods produced from plants. Boron has only two naturally occurring stable isotopes, boron-10 and boron-11.
Compare the abundance of the two naturally occurring isotopes of boron.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Boron-11 is about four times more abundant than boron-10.
• The B-10 is less abundant.
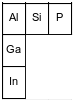
The diagram below represents three elements in Group 13 and three elements in Period 3 and their relative positions on the Periodic Table.
Some elements in the solid phase exist in different forms that vary in their physical properties. For example, at room temperature, red phosphorus has a density of 2.16 g/cm3 and white phosphorus has a density of 1.823 g/cm3.
Identify the element from the diagram that will react with chlorine to form a compound with the general formula XCl4.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Si
• silicon
• element 14
Ethene and hydrogen can react at a faster rate in the presence of the catalyst platinum. The equation below represents a reaction between ethene and hydrogen.
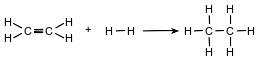
Determine the molar mass of the product.
Allow 1 credit for 30 g/mol, 30. g/mol, or for any value from 30.06 g/mol to 30.1 g/mol, inclusive.
