Topic: Physical Change And Chemical Change
Physical Change And Chemical Change
Which statement describes a chemical change?
(1) Alcohol evaporates.
(2) Water vapor forms snowflakes.
(3) Table salt (NaCl) is crushed into powder.
(4) Glucose (C6H12O6) and oxygen produce CO2 and H2O.
Which processes represent one chemical change and one physical change?
(1) freezing and melting
(2) freezing and vaporization
(3) decomposition and melting
(4) decomposition and combustion
Which process is a chemical change?
(1) evaporating an alcohol
(2) subliming of iodine
(3) melting an ice cube
(4) rusting of iron
Given the key:

Which particle model diagram represents a chemical change?
(1) 
(2) 
(3) 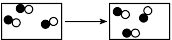
(4) 
Which change results in the formation of different substances?
(1) burning of propane
(2) melting of NaCl(s)
(3) deposition of CO2(g)
(4) solidification of water
Which change is most likely to occur when a molecule of H2 and a molecule of I2 collide with proper orientation and sufficient energy?
(1) a chemical change, because a compound is formed
(2) a chemical change, because an element is formed
(3) a physical change, because a compound is formed
(4) a physical change, because an element is formed
When a sample of CO2(s) becomes CO2(g), there is a change in
(1) bond type
(2) gram-formula mass
(3) molecular polarity
(4) particle arrangement
Which process is a chemical change?
(1) condensation of H2O(g)
(2) synthesis of MgO(s)
(3) evaporation of C2H5OH(ℓ)
(4) sublimation of CO2(s)
The reaction between aluminum and an aqueous solution of copper(II) sulfate is represented by the unbalanced equation below.
Al(s) + CuSO4(aq) → Al2(SO4)3(aq) + Cu(s)
Explain why the equation represents a chemical change.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The products are different substances with different properties from the reactants.
• There is a loss and gain of electrons by substances in the reaction.
Wood is mainly cellulose, a polymer produced by plants. One use of wood is as a fuel in campfires, fireplaces, and wood furnaces. The molecules of cellulose are long chains of repeating units. Each unit of the chain can be represented as C6H10O5. The balanced equation below represents a reaction that occurs when C6H10O5 is burned in air.
C6H10O5 + 6O2 → 6CO2 + 5H2O + heat
Explain, in terms of substances in the reaction, why the equation represents a chemical change.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The products of the reaction are different substances than the reactants.
• The chemical properties of the reactants and the products are different.
• Bonds are broken in the reactants and new bonds are formed in the products.
• Different substances are formed.
Starting as a solid, a sample of a molecular substance is heated, until the entire sample of the substance is a gas. The graph below represents the relationship between the temperature of the sample and the elapsed time.
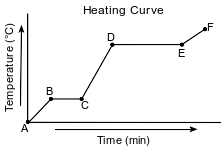
State evidence that indicates the sample undergoes only physical changes during this heating.
Allow 1 credit. Acceptable responses include, but are not limited to:
• No new substance is formed.
• The phase changes do not change the chemical properties of the substance.
Ammonium chloride is dissolved in water to form a 0.10 M NH4Cl(aq) solution. This dissolving process is represented by the equation below.

State evidence that indicates the dissolving of ammonium chloride is an endothermic process.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The process requires heat to dissolve NH4Cl.
• Energy is absorbed as NH4Cl dissolves.
• The energy term is positive on the left side of the equation arrow.
• The heat of reaction is positive.
Seawater contains dissolved salts in the form of ions. Some of the ions found in seawater are Ca2+, Mg2+, K+, Na+, Cl−, HCO3−, and SO42−. An investigation was conducted to determine the concentration of dissolved salts in seawater at one location. A 300.-gram sample of the seawater was placed in an open container. After a week, all the water had evaporated and 10. grams of solid salts remained in the container.
Explain why the evaporation that occurred during the investigation is an endothermic process.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Energy is needed to overcome the intermolecular forces.
• Energy is required to change liquid water to water vapor.
• The heat of vaporization is positive.
Nitrogen dioxide, NO2, is a dark brown gas that is used to make nitric acid and to bleach flour. Nitrogen dioxide has a boiling point of 294 K at 101.3 kPa. In a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, N2O4. This equilibrium is represented by the equation below.
2NO2(g) ⇌ N2O4(g) + 58 kJ
State evidence from the equation that the forward reaction is exothermic.
Allow 1 credit. Acceptable responses include, but are not limited to:
• There are 58 kJ of energy produced by the forward reaction.
• The heat term is on the right side of the equation.
At standard pressure, hydrogen peroxide, H2O2, melts at −0.4°C, boils at 151°C, and is very soluble in water. A bottle of aqueous hydrogen peroxide, H2O2(aq), purchased from a pharmacy has a pressure-releasing cap. Aqueous hydrogen peroxide decomposes at room temperature, as represented by the balanced equation below.
2H2O2(aq) → 2H2O(ℓ) + O2(g) + 196.0 kJ
State evidence that indicates the decomposition of H2O2(aq) is exothermic.
Allow 1 credit. Acceptable responses include, but are not limited to:
• More energy is released than absorbed.
• Heat is a product of the reaction.
