Topic: Polar Bond Polar Molecules
Polar Bond Polar Molecules
Which statement explains why a molecule of CH4 is nonpolar?
(1) The bonds between the atoms in a CH4 molecule are polar.
(2) The bonds between the atoms in a CH4 molecule are ionic.
(3) The geometric shape of a CH4 molecule distributes the charges symmetrically.
(4) The geometric shape of a CH4 molecule distributes the charges asymmetrically.
Which statement explains why a CO2 molecule is nonpolar?
(1) Carbon and oxygen are both nonmetals.
(2) Carbon and oxygen have different electronegativities.
(3) The molecule has a symmetrical distribution of charge.
(4) The molecule has an asymmetrical distribution of charge.
A molecule must be nonpolar if the molecule
(1) is linear
(2) is neutral
(3) has ionic and covalent bonding
(4) has a symmetrical charge distribution
Which phrase describes the molecular polarity and distribution of charge in a molecule of carbon dioxide, CO2?
(1) polar and symmetrical
(2) polar and asymmetrical
(3) nonpolar and symmetrical
(4) nonpolar and asymmetrical
Which phrase describes the distribution of charge and the polarity of a CH4 molecule?
(1) symmetrical and polar
(2) symmetrical and nonpolar
(3) asymmetrical and polar
(4) asymmetrical and nonpolar
Which phrase describes a molecule of CH4, in terms of molecular polarity and distribution of charge?
(1) polar with an asymmetrical distribution of charge
(2) polar with a symmetrical distribution of charge
(3) nonpolar with an asymmetrical distribution of charge
(4) nonpolar with a symmetrical distribution of charge
Which formula represents a nonpolar molecule containing polar covalent bonds?
(1) ![]()
(2) 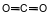
(3) 
(4) 
Given the formula representing a molecule:
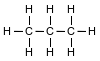
Which statement explains why the molecule is nonpolar?
(1) Electrons are shared between the carbon atoms and the hydrogen atoms.
(2) Electrons are transferred from the carbon atoms to the hydrogen atoms.
(3) The distribution of charge in the molecule is symmetrical.
(4) The distribution of charge in the molecule is asymmetrical.
Which statement describes the charge distribution and the polarity of a CH4 molecule?
(1) The charge distribution is symmetrical and the molecule is nonpolar.
(2) The charge distribution is asymmetrical and the molecule is nonpolar.
(3) The charge distribution is symmetrical and the molecule is polar.
(4) The charge distribution is asymmetrical and the molecule is polar.
Which formula represents a polar molecule?
(1) O2
(2) CO2
(3) NH3
(4) CH4
Which formula represents an asymmetrical molecule?
(1) CH4
(2) CO2
(3) N2
(4) NH3
Which phrase describes the molecular polarity of and the charge distribution in an HCl molecule?
(1) nonpolar with an asymmetrical charge distribution
(2) nonpolar with a symmetrical charge distribution
(3) polar with an asymmetrical charge distribution
(4) polar with a symmetrical charge distribution
A solution of ethylene glycol and water can be used as the coolant in an engine-cooling system. The ethylene glycol concentration in a coolant solution is often given as percent by volume. For example, 100. mL of a coolant solution that is 40.% ethylene glycol by volume contains 40. mL of ethylene glycol diluted with enough water to produce a total volume of 100. mL. The graph below shows the freezing point of coolants that have different ethylene glycol concentrations.
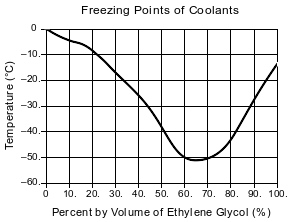
Explain, in terms of the molecular polarity, why ethylene glycol dissolves in water to form a solution.
Allow 1 credit. Acceptable response include, but are not limited to:
• Water molecules and ethylene glycol molecules are both polar.
• Water and the glycol have similar polarities.
Rubbing alcohol is a product available at most pharmacies and supermarkets. One rubbing alcohol solution contains 2-propanol and water. The boiling point of 2-propanol is 82.3°C at standard pressure.
Explain, in terms of charge distribution, why a molecule of the 2-propanol is a polar molecule.
Allow 1 credit. Acceptable responses include, but are not limited to:
• A 2-propanol molecule is polar because it has an asymmetrical distribution of charge.
• The charge distribution is uneven.
• The center of positive charge and the center of negative charge do not coincide.
The Lewis electron-dot diagrams for three substances are shown below.

Explain, in terms of distribution of charge, why a molecule of the substance represented in diagram 3 is nonpolar.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Charge is symmetrically distributed.
• The molecule has uniform charge distribution.
• The centers of positive charge and negative charge coincide.
