Topic: States Of Matter
States Of Matter
Which sample of CO2 has a definite shape and a definite volume?
(1) CO2(aq)
(2) CO2(g)
(3) CO2(ℓ)
(4) CO2(s)
Some physical properties of two samples of iodine-127 at two different temperatures are shown in the table below.

These two samples are two different
(1) mixtures
(2) substances
(3) phases of matter
(4) isotopes of iodine
At 1 atm, equal masses of H2O(s), H2O(ℓ), and H2O(g) have
(1) the same density
(2) the same distance between molecules
(3) different volumes
(4) different percent compositions
A sample of CO2(s) and a sample of CO2(g) differ in their
(1) chemical compositions
(2) empirical formulas
(3) molecular structures
(4) physical properties
In which 1.0-gram sample are the particles arranged in a crystal structure?
(1) CaCl2(s)
(2) C2H6(g)
(3) CH3OH(ℓ)
(4) CaI2(aq)
Which sample of matter has a crystal structure?
(1) Hg(ℓ)
(2) H2O(ℓ)
(3) NaCl(s)
(4) CH4(g)
One mole of liquid water and one mole of solid water have different
(1) masses
(2) properties
(3) empirical formulas
(4) gram-formula masses
A gas changes directly to a solid during
(1) fusion
(2) deposition
(3) saponification
(4) decomposition
Which sample of matter sublimes at room temperature and standard pressure?
(1) Br2(ℓ)
(2) Cl2(g)
(3) CO2(s)
(4) SO2(aq)
Which equation represents sublimation?
(1) Hg(ℓ) → Hg(s)
(2) H2O(s) → H2O(g)
(3) NH3(g) → NH3(ℓ)
(4) CH4(ℓ) → CH4(g)
Starting as a gas at 206°C, a sample of a substance is allowed to cool for 16 minutes. This process is represented by the cooling curve below.

Using the key below, draw two particle diagrams to represent the two phases of the sample at minute 4. Your response must include at least six particles for each diagram.
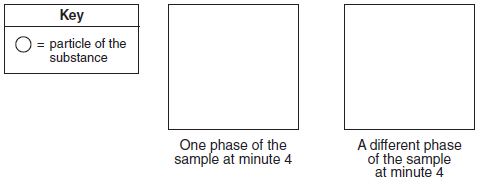
Allow 1 credit. Particles of the gas must be drawn farther apart than particles of the liquid.
• Example of a 1-credit response:
• chem12012-rg_g2.png
Paintball is a popular recreational activity that uses a metal tank of compressed carbon dioxide or nitrogen to launch small capsules of paint. A typical tank has a volume of 508 cubic centimeters. A 340.-gram sample of carbon dioxide is added to the tank before it is used for paintball. At 20.°C, this tank contains both CO2(g) and CO2(ℓ). After a paintball game, the tank contains only CO2(g).
In the box below, use the key to draw a particle diagram to represent the two phases of CO2 in a newly filled tank. Your response must include at least six molecules of CO2 in each phase.
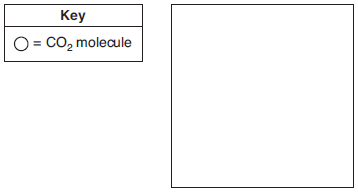
Allow 1 credit. Molecules of the gas must be drawn farther apart than the molecules of the liquid.
• Examples of 1-credit responses:
• chem62014-rg_g3.png
At standard pressure, water has unusual properties that are due to both its molecular structure and intermolecular forces. For example, although most liquids contract when they freeze, water expands, making ice less dense than liquid water. Water has a much higher boiling point than most other molecular compounds having a similar gram-formula mass.
Explain why H2O(s) floats on H2O(ℓ) when both are at 0ºC.
Allow 1 credit. Acceptable responses include, but are not limited to:
• When water freezes it expands, making H2O(s) less dense than H2O(ℓ).
• The distance between the H2O molecules is greater in the solid phase.
• The density of liquid water is greater.
• The density of ice is less.
A technician recorded data for two properties of Period 3 elements. The data are shown in the table below.
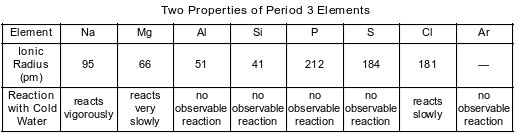
State the phase of chlorine at 281 K and 101.3 kPa.
Allow 1 credit for gas or (g).
In the late 1800s, Dmitri Mendeleev developed a periodic table of the elements known at that time. Based on the pattern in his periodic table, he was able to predict properties of some elements that had not yet been discovered. Information about two of these elements is shown in the table below.

Identify the phase of Ea at 310. K.
Allow 1 credit for liquid or (ℓ).
