Topic: Table B Physical Constants For Water
Table B Physical Constants For Water
What is the total amount of heat required to vaporize 1.00 gram of H2O(ℓ) at 100.°C and 1 atmosphere?
(1) 4.18 J
(2) 334 J
(3) 373 J
(4) 2260 J
What is the amount of heat energy released when 50.0 grams of water is cooled from 20.0°C to 10.0°C?
(1) 5.00 × 102 J
(2) 2.09 × 103 J
(3) 1.67 × 105 J
(4) 1.13 × 106 J
A 100.-gram sample of H2O(ℓ) at 22.0°C absorbs 8360 joules of heat. What will be the final temperature of the water?
(1) 18.3°C
(2) 20.0°C
(3) 25.7°C
(4) 42.0°C
At standard pressure, the total amount of heat required to completely vaporize a 100.-gram sample of water at its boiling point is
(1) 2.26 × 10 J
(2) 2.26 × 102 J
(3) 2.26 × 103 J
(4) 2.26 × 105 J
What is the total amount of heat required to completely melt 347 grams of ice at its melting point?
(1) 334 J
(2) 1450 J
(3) 116 000 J
(4) 784 000 J
What is the amount of heat, in joules, required to increase the temperature of a 49.5-gram sample of water from 22°C to 66°C?
(1) 2.2 × 103 J
(2) 4.6 × 103 J
(3) 9.1 × 103 J
(4) 1.4 × 104 J
What is the amount of heat energy absorbed when 40.0 grams of water is heated from 10.0°C to 30.0°C?
(1) 1.67 × 103 J
(2) 3.34 × 103 J
(3) 5.02 × 103 J
(4) 2.67 × 105 J
What is the amount of heat released by 1.00 gram of liquid water at 0°C when it changes to 1.00 gram of ice at 0°C?
(1) 4.18 J
(2) 273 J
(3) 334 J
(4) 2260 J
Fruit growers in Florida protect oranges when the temperature is near freezing by spraying water on them. It is the freezing of the water that protects the oranges from frost damage. When H2O(ℓ) at 0°C changes to H2O(s) at 0°C, heat energy is released. This energy helps to prevent the temperature inside the orange from dropping below freezing, which could damage the fruit. After harvesting, oranges can be exposed to ethene gas, C2H4, to improve their color.
Determine the quantity of heat released when 2.00 grams of H2O(ℓ) freezes at 0°C.
Allow 1 credit for 668 J or –668 J.
A 100.-gram sample of liquid water is heated from 20.0°C to 50.0°C. Enough KClO3(s) is dissolved in the sample of water at 50.0°C to form a saturated solution.
Using the information on Table B, determine the amount of heat absorbed by the water when the water is heated from 20.0°C to 50.0°C.
Allow 1 credit for 12 500 J or any value from 12 500 J to 13 000 J, inclusive.
During a laboratory activity, a student heats a beaker containing 120.0 grams of water as shown in the diagram below.
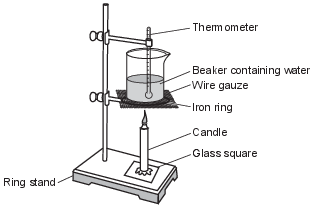
The table below shows the mass of the water and the temperature of the water before and after heating. During this laboratory activity, appropriate safety equipment is used and safety procedures are followed.

Show a numerical setup for calculating the amount of heat, in joules, absorbed by the water in the beaker as a result of the burning candle. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• (120.0 g)(4.18 J/g•K)(86.0°C - 23.0°C)
• (120.0 g)(4.18 J/g•K)(359 K - 296 K)
• (120)(4.2)(63)
