Topic: Table E Selected Polyatomic Ions
Table E Selected Polyatomic Ions
What is the chemical formula for zinc carbonate?
(1) ZnCO3
(2) Zn(CO3)2
(3) Zn2CO3
(4) Zn3CO2
Given the incomplete equation representing a reaction:
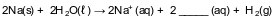
What is the formula of the missing product?
(1) O2−
(2) O2
(3) OH−
(4) OH
Which formula represents ammonium nitrate?
(1) NH4NO3
(2) NH4NO2
(3) NH4(NO3)2
(4) NH4(NO2)2
What is the chemical name of the compound NH4SCN?
(1) ammonium thiocyanate
(2) ammonium cyanide
(3) nitrogen hydrogen cyanide
(4) nitrogen hydrogen sulfate
Which polyatomic ion is found in the compound represented by the formula NaHCO3?
(1) acetate
(2) hydrogen carbonate
(3) hydrogen sulfate
(4) oxalate
Which positive ion must be present in an aqueous solution of an Arrhenius acid?
(1) H3O+
(2) Na+
(3) NH4+
(4) Rb+
A total of 1.4 moles of sodium nitrate is dissolved in enough water to make 2.0 liters of an aqueous solution. The gram-formula mass of sodium nitrate is 85 grams per mole.
Write the chemical formula for the solute in the solution.
Allow 1 credit for NaNO3.
A company produces a colorless vinegar that is 5.0% HC2H3O2 in water. Using thymol blue as an indicator, a student titrates a 15.0-milliliter sample of the vinegar with 43.1 milliliters of a 0.30 M NaOH(aq) solution until the acid is neutralized.
Identify the negative ion in the NaOH(aq) used in this titration.
Allow 1 credit for OH− or hydroxide.
In a titration using a pH meter, 16.0 milliliters of 0.18 M NaOH(aq) exactly neutralizes a 24.0-milliliter sample of HCl(aq) in a flask. During this laboratory activity, appropriate safety equipment was used and safety procedures were followed.
Identify the negative ion in the NaOH(aq) used in the titration. [1]
Allow 1 credit for OH− or hydroxide or hydroxide ion.
• Note: Do not allow credit for OH or hydroxyl or hydroxyl ion.
Four different samples of NaNO3(aq) are each evaporated to dryness. The solution volume and mass of the dry NaNO3(s) of each sample are recorded in the table below.
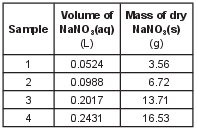
The number of moles of NaNO3(s) of each sample was then calculated and used to produce the graph below.
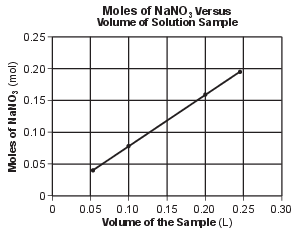
Write a chemical name for NaNO3. [1]
Allow 1 credit for sodium nitrate.
