Topic: Table F Solubility Guidelines For Aqueous Solutions
Table F Solubility Guidelines For Aqueous Solutions
Which compound is soluble in water?
(1) PbS
(2) BaS
(3) Na2S
(4) Fe2S3
Which compounds are electrolytes?
(1) C2H5OH and H2SO4
(2) C2H5OH and CH4
(3) KOH and H2SO4
(4) KOH and CH4
Which substance is an electrolyte?
(1) C6H12O6(s)
(2) C2H5OH(ℓ)
(3) NaOH(s)
(4) H2(g)
According to Table F, which ions combine with chloride ions to form an insoluble compound?
(1) Fe2+ ions
(2) Ca2+ ions
(3) Li+ ions
(4) Ag+ ions
Which compound is an electrolyte?
(1) CH3CHO
(2) CH3OCH3
(3) CH3COOH
(4) CH3CH2CH3
A 1-gram sample of a compound is added to 100 grams of H2O(ℓ) and the resulting mixture is then thoroughly stirred. Some of the compound is then separated from the mixture by filtration. Based on Table F, the compound could be
(1) AgCl
(2) CaCl2
(3) NaCl
(4) NiCl2
Which ion combines with Ba2+ to form a compound that is most soluble in water?
(1) S2−
(2) OH−
(3) CO32−
(4) SO42−
Which compound is an electrolyte?
(1) H2O
(2) C2H6
(3) H3PO4
(4) CH3OH
Which formula represents an electrolyte?
(1) H2O
(2) CCl4
(3) H2SO4
(4) C6H12O6
In a titration, 50.0 milliliters of 0.026 M HCl(aq) is neutralized by 38.5 milliliters of KOH(aq).
Complete the equation below for the neutralization by writing the formula of the missing product.

Allow 1 credit. Acceptable responses include, but are not limited to:
• KCl
• ClK
• K+(aq) + Cl−(aq)
• K+ + Cl−
Natural gas and coal are two fuels burned to produce energy. Natural gas consists of approximately 80% methane, 10% ethane, 4% propane, 2% butane, and other components.
The burning of coal usually produces sulfur dioxide, SO2(g), and sulfur trioxide, SO3(g), which are major air pollutants. Both SO2(g) and SO3(g) react with water in the air to form acids.
Complete the equation below representing the reaction of sulfur trioxide with water to produce sulfuric acid, by writing the formula of the missing reactant and the formula of the missing product.

Allow 1 credit. The order of the elements in each compound may vary.
• SO3(g) + H2O(ℓ) → H2SO4(aq)
A student prepares two 141-gram mixtures, A and B. Each mixture consists of NH4Cl, sand, and H2O at 15°C. Both mixtures are thoroughly stirred and allowed to stand. The mass of each component used to make the mixtures is listed in the data table below.
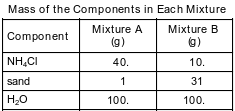
Determine the temperature at which all of the NH4Cl in mixture A dissolves to form a saturated solution.
Allow 1 credit for any value from 23ºC to 26ºC, inclusive.
In a titration, 20.0 milliliters of 0.15 M HCl(aq) is exactly neutralized by 18.0 milliliters of KOH(aq).
Complete the equation below for the neutralization reaction by writing the formula of each product.
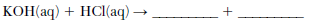
Allow 1 credit. Acceptable responses include, but are not limited to:
• H2O(ℓ ) and KCl(aq)
• KCl and HOH
The elements in Group 17 are called halogens. The word “halogen” is derived from Greek and means “salt former.”
Based on Table F, identify one ion that reacts with iodide ions in an aqueous solution to form an insoluble compound.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Hg 22+
• Pb2+
• mercury(I) ion
• silver
Some compounds of silver are listed with their chemical formulas in the table below.
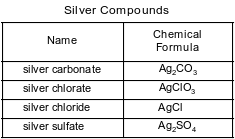
Identify the silver compound in the table that is most soluble in water.
Allow 1 credit for AgClO3 or silver chlorate.
