Topic: Table G Solubility Curves At Standard Pressure
Table G Solubility Curves At Standard Pressure
Based on Table G, which compound has the greatest solubility in 100. grams of water at 10.°C?
(1) HCl
(2) NaCl
(3) KCl
(4) NH4Cl
Based on Table G, which compound is less soluble in water as the temperature increases from 0°C to 100°C?
(1) KNO3
(2) NH3
(3) KClO3
(4) NH4Cl
Based on Table G, which solute sample in 100.g of water at 40.°C can produce a solution equilibrium in a closed system?
(1) 10. g KClO3
(2) 25 g NaCl
(3) 45 g KCl
(4) 55 g KNO3
Ammonium chloride is dissolved in water to form a 0.10 M NH4Cl(aq) solution. This dissolving process is represented by the equation below.

Determine the minimum mass of NH4Cl(s) required to produce a saturated solution in 100. grams of water at 40.°C.
Allow 1 credit for 47 g ± 1 g.
The compounds KNO3 and NaNO3 are soluble in water.
Compare the boiling point of a NaNO3 solution at standard pressure to the boiling point of water at standard pressure.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The boiling point of the NaNO3 solution is higher than the boiling point of water.
• lower for H2O
Baking soda, NaHCO3, can be commercially produced during a series of chemical reactions called the Solvay process. In this process, NH3(aq), NaCl(aq), and other chemicals are used to produce NaHCO3(s) and NH4Cl(aq).
To reduce production costs, NH3(aq) is recovered from NH4Cl(aq) through a different series of reactions. This series of reactions can be summarized by the overall reaction represented by the unbalanced equation below.
NH4Cl(aq) + CaO(s) → NH3(aq) + H2O(ℓ) + CaCl2(aq)
Determine the mass of NH4Cl that must be dissolved in 100. grams of H2O to produce a saturated solution at 70.°C.
Allow 1 credit for any value from 61 g to 63 g, inclusive.
In a laboratory investigation, a student is given a sample that is a mixture of 3.0 grams of NaCl(s) and 4.0 grams of sand, which is mostly SiO2(s). The purpose of the investigation is to separate and recover the compounds in the sample. In the first step, the student places the sample in a 250-mL flask. Then, 50. grams of distilled water are added to the flask, and the contents are thoroughly stirred. The mixture in the flask is then filtered, using the equipment represented by the diagram below.
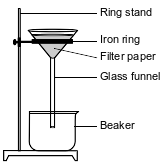
Based on Table G, state evidence that all of the NaCl(s) in the flask would dissolve in the distilled water at 20.°C.
Allow 1 credit. Acceptable responses include, but are not limited to:
• According to Table G, the salt solution is unsaturated.
• The 3.0 g of salt dissolved in 50. g of H2O has a concentration less than the solubility of NaCl on Table G at 20.°C.
• Table G indicates that the solubility of NaCl is greater than the amount in the sample.
What is the mass of KNO3(s) that must dissolve in 100. grams of water to form a saturated solution at 50.°C?
Allow 1 credit for 84 g ± 2 g.
Determine the mass of KNO3 that dissolves in 100. grams of water at 40.°C to produce a saturated solution.
Allow 1 credit for 64 g, or any value from 62 g to 66 g, inclusive.
A solution is made by dissolving 70.0 grams of KNO3(s) in 100. grams of water at 50.°C and standard pressure.
Determine the number of additional grams of KNO3 that must dissolve to make this solution saturated.
Allow 1 credit for any value from 12 g to 16 g, inclusive.
Using Table G, determine the minimum mass of NaCl that must be dissolved in 200. grams of water to produce a saturated solution at 90.°C.
Allow 1 credit for any value from 78 g to 82 g inclusive.
A saturated solution of sulfur dioxide is prepared by dissolving SO2(g) in 100. g of water at 10.°C and standard pressure.
Based on Table G, state the general relationship between solubility and temperature of an aqueous SO2 solution at standard pressure.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The solubility at 1 atm increases as the temperature decreases.
• As the temperature of the solution increases, the solubility of SO2 decreases.
• At lower temperatures, more SO2 can dissolve.
A 100.-gram sample of liquid water is heated from 20.0°C to 50.0°C. Enough KClO3(s) is dissolved in the sample of water at 50.0°C to form a saturated solution.
Based on Table G, determine the mass of KClO3(s) that must dissolve to make a saturated solution in 100. g of H2O at 50.0°C.
Allow 1 credit for any value from 20. g to 23 g, inclusive.
A sample of normal rainwater has a pH value of 5.6 due to dissolved carbon dioxide gas from the atmosphere. Acid rain is formed when other gases, such as sulfur dioxide, dissolve in rainwater, which can result in lake water with a pH value of 4.6. The equation below represents the reaction of water with SO2(g).
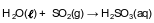
Based on Table G, describe what happens to the solubility of SO2(g) as the temperature increases from 10.°C to 30.°C at standard pressure. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• As the water temperature increases, the solubility of sulfur dioxide decreases.
• The solubility of SO2 decreases.
• The SO2(g) becomes less soluble.
