Topic: Table I Heats Of Reaction At 101.3 Kpa And 298 K
Table I Heats Of Reaction At 101.3 Kpa And 298 K
Given the balanced equation representing a reaction occurring at 101.3 kilopascals and 298 K:
2H2(g) + O2(g) → 2H2O(ℓ) + energy
What is the net amount of energy released when one mole of H2O(ℓ) is produced?
(1) 241.8 kJ
(2) 285.8 kJ
(3) 483.6 kJ
(4) 571.6 kJ
At 101.3 kPa and 298 K, what is the total amount of heat released when one mole of aluminum oxide, Al2O3(s), is formed from its elements?
(1) 393.5 kJ
(2) 837.8 kJ
(3) 1676 kJ
(4) 3351 kJ
According to Table I, which equation represents a change resulting in the greatest quantity of energy released?
(1) 2C(s) + 3H2(g) → C2H6(g)
(2) 2C(s) + 2H2(g) → C2H4(g)
(3) N2(g) + 3H2(g) → 2NH3(g)
(4) N2(g) + O2(g) → 2NO(g)
Based on Table I, what is the ∆H value for the production of 1.00 mole of NO2(g) from its elements at 101.3 kPa and 298 K?
(1) +33.2 kJ
(2) −33.2 kJ
(3) +132.8 kJ
(4) −132.8 kJ
Based on Table I, which equation represents a reaction with the greatest difference between the potential energy of the products and the potential energy of the reactants?
(1) 4Al(s) + 3O2(g) → 2Al2O3(s)
(2) 2H2(g) + O2(g) → 2H2O(ℓ)
(3) C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(ℓ)
(4) C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(ℓ)
Based on Table I, which equation represents conservation of mass and energy?
(1) CH4(g) + O2(g) + 890.4 kJ → CO2(g) + H2O(ℓ)
(2) CH4(g) + O2(g) → CO2(g) + H2O(ℓ) + 890.4 kJ
(3) CH4(g) + 2O2(g) + 890.4 kJ → CO2(g) + 2H2O(ℓ)
(4) CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ) + 890.4 kJ
Ammonium chloride is dissolved in water to form a 0.10 M NH4Cl(aq) solution. This dissolving process is represented by the equation below.

State evidence that indicates the dissolving of ammonium chloride is an endothermic process.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The process requires heat to dissolve NH4Cl.
• Energy is absorbed as NH4Cl dissolves.
• The energy term is positive on the left side of the equation arrow.
• The heat of reaction is positive.
In a laboratory activity, each of four different masses of KNO3(s) is placed in a separate test tube that contains 10.0 grams of H2O at 25°C.
When each sample is first placed in the water, the temperature of the mixture decreases. The mixture in each test tube is then stirred while it is heated in a hot water bath until all of the KNO3(s) is dissolved. The contents of each test tube are then cooled to the temperature at which KNO3 crystals first reappear. The procedure is repeated until the recrystallization temperatures for each mixture are consistent, as shown in the table below.

Based on Table I, explain why there is a decrease in temperature when the KNO3(s) was first dissolved in the water.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The solution would decrease in temperature because the dissolving of KNO3(s) is endothermic.
• The heat of solution is positive, which means the mixture would decrease in temperature.
• The ∆H is + 34.89 kJ, so KNO3(s) requires energy to dissolve.
In the early 1900s, scientists developed a process to produce ammonia from hydrogen and atmospheric nitrogen on an industrial scale. The balanced equation below represents this reaction.
N2(g) + 3H2(g) → 2NH3(g) + 91.8 kJ
At room temperature, the reaction occurs at a very slow rate. Therefore, this process takes place in a special reaction vessel at high temperature and high pressure. A catalyst is used to increase the rate of the production of ammonia. The reaction gases are cooled to remove the ammonia as a liquid and the remaining gases are sent back to the reaction vessel.
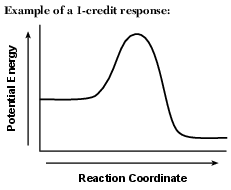
Using the axes shown below, draw a potential energy diagram for the reaction. [1]
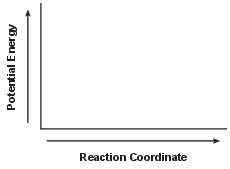
Allow 1 credit for showing that the PE of the products is lower than the PE of the reactants.
• 
During a laboratory activity, a student dissolves 20.0 grams of solid ammonium chloride, NH4Cl(s), in 100.0 grams of water at 25°C. After thorough stirring, no undissolved NH4Cl(s) remains. During this laboratory activity, appropriate safety equipment is used and safety procedures are followed.
State evidence from Table I that indicates that this dissolving process is endothermic. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• The nH for this dissolving is 114.78 kJ/mol.
• The heat of solution is positive.
Hydrogen gas and iodine gas can combine in a reversible reaction to form hydrogen iodide gas. The equation below represents this system at equilibrium in a sealed, rigid container.
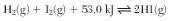
State evidence from the equation that the forward reaction is endothermic. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• The equation shows energy on the reactant side.
• Energy is on the left side of the equation.
• The 53 kJ is on the left side.
• heat term on reactant side
