Topic: Table J Activity Series
Table J Activity Series
Which metal will spontaneously react with Zn2+(aq), but will not spontaneously react with Mg2+(aq)?
(1) Mn(s)
(2) Cu(s)
(3) Ni(s)
(4) Ba(s)
Which element reacts spontaneously with 1.0 M HCl(aq) at room temperature?
(1) copper
(2) gold
(3) silver
(4) zinc
Which ion is most easily reduced?
(1) Zn2+
(2) Mg2+
(3) Co2+
(4) Ca2+
Which metal reacts spontaneously with Sr2+ ions?
(1) Ca(s)
(2) Co(s)
(3) Cs(s)
(4) Cu(s)
Which metal reacts spontaneously with NiCl2(aq)?
(1) Au(s)
(2) Cu(s)
(3) Sn(s)
(4) Zn(s)
Which equation represents a spontaneous reaction?
(1) Ca + Ba2+ → Ca2+ + Ba
(2) Co + Zn2+ → Co2+ + Zn
(3) Fe + Mg2+ → Fe2+ + Mg
(4) Mn + Ni2+ → Mn2+ + Ni
Based on Table J, which metal is more active than tin, but less active than zinc?
(1) Ag
(2) Cr
(3) Cs
(4) Mn
Based on Table J, which ionic equation represents a spontaneous reaction that can occur in a voltaic cell?
(1) Fe(s) + Mg2+(aq) → Fe2+(aq) + Mg(s)
(2) Fe(s) + Mg(s) → Fe2+(aq) + Mg2+(aq)
(3) Fe2+(aq) + Mg2+(aq) → Fe(s) + Mg(s)
(4) Fe2+(aq) + Mg(s) → Fe(s) + Mg2+(aq)
A student constructs an electrochemical cell during a laboratory investigation. When the switch is closed, electrons flow through the external circuit. The diagram and equation below represent this cell and the reaction that occurs.

State the direction of electron flow through the wire when the switch is closed.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Electrons flow from the Al electrode to the Ni electrode.
• Electrons move left to right through the wire.
An operating voltaic cell has zinc and iron electrodes. The cell and the unbalanced ionic equation representing the reaction that occurs in the cell are shown below.

Identify one metal from Table J that is more easily oxidized than Zn.
Allow 1 credit for the symbol or name of any metal listed above Zn on Table J.
A small digital clock can be powered by a battery made from two potatoes and some household materials. The “potato clock” battery consists of two cells connected in a way to produce enough electricity to allow the clock to operate. In each cell, zinc atoms react to form zinc ions. Hydrogen ions from phosphoric acid in the potatoes react to form hydrogen gas. The labeled diagram and balanced ionic equation below show the reaction, the materials, and connections necessary to make a “potato clock” battery.

State the direction of electron flow in wire A as the two cells operate.
Allow 1 credit. Acceptable responses include, but are not limited to:
• from the zinc-coated nail to the copper rod
• from Zn to Cu
• left to right
A student constructs an electrochemical cell during a laboratory investigation. When the switch is closed, electrons flow through the external circuit. The diagram and ionic equation below represent this cell and the reaction that occurs.

State, in terms of the Cu(s) electrode and the Zn(s) electrode, the direction of electron flow in the external circuit when the cell operates.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Electrons flow from the zinc electrode to the copper electrode through the wires and voltmeter.
• The e− flow is from Zn to Cu.
Stamping an identification number into the steel frame of a bicycle compresses the crystal structure of the metal. If the number is filed off, there are scientific ways to reveal the number.
One method is to apply aqueous copper(II) chloride to the number area. The Cu2+ ions react with some iron atoms in the steel frame, producing copper atoms that show the pattern of the number. The ionic equation below represents this reaction.
Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
Another method is to apply hydrochloric acid to the number area. The acid reacts with the iron, producing bubbles of hydrogen gas. The bubbles form faster where the metal was compressed, so the number becomes visible. The equation below represents this reaction.
2HCl(aq) + Fe(s) → FeCl2(aq) + H2(g)
Explain why the Fe atoms in the bicycle frame react with the Cu2+ ions.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Fe oxidizes in the presence of Cu2+ ions.
• Iron is a more active metal than copper.
• Cu2 ions act as an oxidizing agent.
• Fe is above Cu on Table J.
During a laboratory activity, appropriate safety equipment is used and safety procedures are followed. A student constructs a voltaic cell with magnesium and copper electrodes. The diagram and net ionic equation below represent this cell and the reaction that occurs.
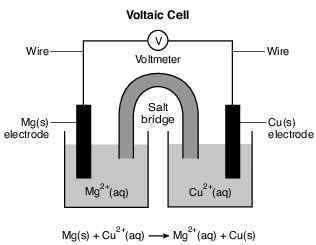
Identify one metal from Table J that is more easily oxidized than Mg. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• Na
• calcium
• Sr
• barium
• Cs
• K
• Rb
• Li
When solid copper is placed in an aqueous silver nitrate solution, a reaction occurs, as represented by the equation below.
Cu(s) + 2AgNO3(aq) → 2Ag(s) + Cu(NO3)2(aq)
Based on Table J, state why Cu(s) reacts spontaneously with Ag1(aq). [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• Copper is more active than silver.
• Silver is less active than copper.
• Cu is above Ag on Table J.
• Note: Do not allow credit for Cu is more active than Ag+.
