Topic: Table K Common Acids
Table K Common Acids
Which compound yields H+ ions as the only positive ions in an aqueous solution?
(1) KOH
(2) NaOH
(3) CH3OH
(4) CH3COOH
Which substance is an Arrhenius acid?
(1) H2
(2) HCl
(3) KCl
(4) NH3
Which compounds are classified as Arrhenius acids?
(1) HCl and NaOH
(2) HNO3 and NaCl
(3) NH3 and H2CO3
(4) HBr and H2SO4
Which formula represents an Arrhenius acid?
(1) KCl
(2) HCl
(3) NH3
(4) KOH
In a laboratory activity, a student titrates a 20.0-milliliter sample of HCl(aq) using 0.025 M NaOH(aq). In one of the titration trials, 17.6 milliliters of the base solution exactly neutralizes the acid sample.
Identify the positive ion in the sample of HCl(aq).
Allow 1 credit. Acceptable responses include, but are not limited to:
• hydronium ion
• H3O+
• hydronium
• H+
• hydrogen ion
• H3O+(aq)
• hydrogen
• H+(aq)
• proton
A student is to determine the concentration of an NaOH(aq) solution by performing two different titrations. In a first titration, the student titrates 25.0 mL of 0.100 M H2SO4(aq) with NaOH(aq) of unknown concentration.
In a second titration, the student titrates 25.0 mL of 0.100 M HCl(aq) with a sample of the NaOH(aq). During this second titration, the volume of the NaOH(aq) added and the corresponding pH value of the reaction mixture is measured. The graph below represents the relationship between pH and the volume of the NaOH(aq) added for this second titration.
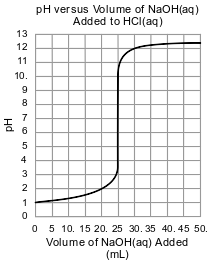
Identify the positive ion present in the H2SO4(aq) solution before the titration.
Allow 1 credit. Acceptable responses include, but are not limited to:
• hydronium ion
• H3O+
• hydronium
• H+
• hydrogen ion
• H3O+(aq)
• hydrogen
• H+ (aq)
• proton
