Topic: Table M Common Acid Base Indicators
Table M Common Acid Base Indicators
Phenolphthalein is pink in an aqueous solution having a pH of
(1) 5
(2) 2
(3) 7
(4) 12
What is the color of the indicator thymol blue in a solution that has a pH of 11?
(1) red
(2) blue
(3) pink
(4) yellow
What is the color of bromcresol green indicator in a solution with a pH value of 2.0?
(1) blue
(2) green
(3) red
(4) yellow
Which indicator is blue in a solution that has a pH value of 7.0?
(1) bromcresol green
(2) methyl orange
(3) phenolphthalein
(4) thymol blue
The active ingredient in the pain reliever aspirin is acetylsalicylic acid. This compound can be produced by reacting salicylic acid with acetic acid. The label of one aspirin bottle indicates that the accepted mass of acetylsalicylic acid in each tablet is 325 milligrams.
In a laboratory, an aspirin tablet is crushed and mixed with water to dissolve all of the acetylsalicylic acid. The measured pH of the resulting solution is 3.0.
State the color of methyl orange indicator after the indicator is placed in the solution.
Allow 1 credit for red.
Baking soda, NaHCO3, can be commercially produced during a series of chemical reactions called the Solvay process. In this process, NH3(aq), NaCl(aq), and other chemicals are used to produce NaHCO3(s) and NH4Cl(aq).
To reduce production costs, NH3(aq) is recovered from NH4Cl(aq) through a different series of reactions. This series of reactions can be summarized by the overall reaction represented by the unbalanced equation below.
NH4Cl(aq) + CaO(s) → NH3(aq) + H2O(ℓ) + CaCl2(aq)
State the color of bromcresol green in a sample of NH3(aq).
Allow 1 credit for blue.
The diagram below shows typical pH values found in four parts of the human digestive system. In the small intestine, the enzyme lipase acts as a catalyst, increasing the rate of fat digestion.

What is the color of thymol blue at the pH of the small intestine?
Allow 1 credit for yellow.
A scientist bubbled HCl(g) through a sample of H2O(ℓ). This process is represented by the balanced equation below.
H2O(ℓ) + HCl(g) → H3O+(aq) + Cl−(aq)
The scientist measured the pH of the liquid in the flask before and after the gas was bubbled through the water. The initial pH value of the water was 7.0 and the final pH value of the solution was 3.0.
What would be the color of bromcresol green if it had been added to the water in the flask before any of the HCl(g) was bubbled through the water?
Allow 1 credit for blue.
Vinegar is a commercial form of acetic acid, HC2H3O2(aq). One sample of vinegar has a pH value of 2.4.
State the color of bromthymol blue indicator in a sample of the commercial vinegar.
Allow 1 credit for yellow.
Carbon dioxide is slightly soluble in seawater. As carbon dioxide levels in the atmosphere increase, more CO2 dissolves in seawater, making the seawater more acidic because carbonic acid, H2CO3(aq), is formed.
Seawater also contains aqueous calcium carbonate, CaCO3(aq), which is used by some marine organisms to make their hard exoskeletons. As the acidity of the sea water changes, the solubility of CaCO3 also changes, as shown in the graph below.
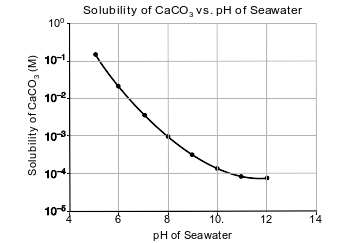
State the color of bromcresol green in a sample of seawater in which the CaCO3 solubility is 10−2 M.
Allow 1 credit for blue.
A company produces a colorless vinegar that is 5.0% HC2H3O2 in water. Using thymol blue as an indicator, a student titrates a 15.0-milliliter sample of the vinegar with 43.1 milliliters of a 0.30 M NaOH(aq) solution until the acid is neutralized.
Based on Table M, what is the color of the indicator in the vinegar solution before any base is added?
Allow 1 credit for yellow.
A student is to determine the concentration of an NaOH(aq) solution by performing two different titrations. In a first titration, the student titrates 25.0 mL of 0.100 M H2SO4(aq) with NaOH(aq) of unknown concentration.
In a second titration, the student titrates 25.0 mL of 0.100 M HCl(aq) with a sample of the NaOH(aq). During this second titration, the volume of the NaOH(aq) added and the corresponding pH value of the reaction mixture is measured. The graph below represents the relationship between pH and the volume of the NaOH(aq) added for this second titration.
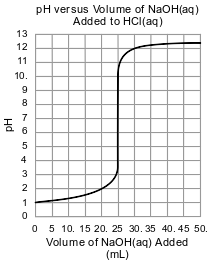
State the color of phenolphthalein indicator if it were added after the HCl(aq) was titrated with 50. mL of NaOH(aq).
Allow 1 credit for pink.
A 30.0-milliliter sample of HCl(aq) was exactly neutralized by 18.0 milliliters of 0.10 M KOH(aq). During this laboratory activity, appropriate safety equipment was used and safety procedures were followed.
State the color of bromcresol green indicator if it were added to a sample of the 0.10 M KOH(aq). [1]
Allow 1 credit for blue.
A sample of normal rainwater has a pH value of 5.6 due to dissolved carbon dioxide gas from the atmosphere. Acid rain is formed when other gases, such as sulfur dioxide, dissolve in rainwater, which can result in lake water with a pH value of 4.6. The equation below represents the reaction of water with SO2(g).
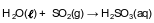
State the color of methyl orange in a sample of normal rainwater. [1]
Allow 1 credit for yellow.
In a laboratory activity, a student measures the pH values of four household liquids and distilled water, as shown in the table below. During this laboratory activity, appropriate safety equipment is used and safety procedures are followed.

State the color of bromcresol green after the indicator is added to a sample of lemon juice. [1]
Allow 1 credit for yellow.
