Topic: Table P Organic Prefixes
Table P Organic Prefixes
Given the formula representing a compound:

What is a chemical name of this compound?
(1) 2-pentene
(2) 2-pentyne
(3) 3-pentene
(4) 3-pentyne
What is the name of the compound with the formula CH3CH2CH2NH2?
(1) 1-propanol
(2) 1-propanamine
(3) propanal
(4) propanamide
Given the formula representing a compound:

What is an IUPAC name for this compound?
(1) ethyl propanoate
(2) propyl ethanoate
(3) 3-hexanone
(4) 4-hexanone
What is the chemical name for the compound CH3CH2CH2CH3?
(1) butane
(2) butene
(3) decane
(4) decene
Given the formula representing a compound:
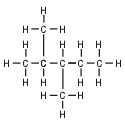
What is a chemical name of this compound?
(1) 2,3,3-trimethylbutane
(2) 2-methyl-2-ethylbutane
(3) 2,3-dimethylpentane
(4) 2,3-ethylpentane
Given the formula representing a compound:
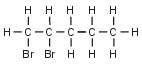
What is a chemical name of this compound?
(1) 1,1-dibromopentane
(2) 2,2-dibromopentane
(3) 1,2-dibromopentane
(4) 4,5-dibromopentane
Crude oil, primarily a mixture of hydrocarbons, is separated into useful components in a fractionating tower. At the bottom of the tower, the crude oil is heated to about 400°C. The gases formed rise and cool. Most of the gases condense and are collected as liquid fractions. The table below shows the temperature ranges for collecting various hydrocarbon fractions.
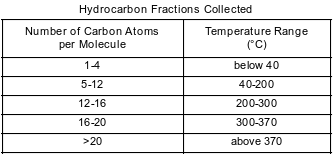
State the temperature range for the fraction collected that contains octane molecules.
Allow 1 credit for 40°C to 200°C. Significant figures do not need to be shown.
Water, H2O, and hexane, C6H14, are commonly used as laboratory solvents because they have different physical properties and are able to dissolve different types of solutes. Some physical properties of water and hexane are listed on the table below.

Explain, in terms of molecular formulas and structural formulas, why 2,2-dimethylbutane is an isomer of hexane. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• The hexane and the 2,2-dimethylhexane have the same molecular formula but have different structural formulas.
• Both molecules have the same number of C atoms and the same number of H atoms but have a different arrangement of atoms.
• Both compounds are C6H14, but have different structures.
