Topic: Types Of Organic Reactions
Types Of Organic Reactions
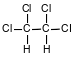
Which formula represents the product of the addition reaction between ethene and chlorine, Cl2?
(1) 
(2) 
(3) 
(4) 
Which term identifies a type of organic reaction?
(1) deposition
(2) distillation
(3) esterification
(4) sublimation
Which reaction produces ethanol?
(1) combustion
(2) esterification
(3) fermentation
(4) polymerization
Two types of organic reactions are
(1) deposition and saponification
(2) deposition and transmutation
(3) polymerization and saponification
(4) polymerization and transmutation
Which type of reaction includes esterification and polymerization?
(1) decomposition
(2) neutralization
(3) organic
(4) nuclear
A reaction between an alcohol and an organic acid is classified as
(1) esterification
(2) fermentation
(3) saponification
(4) substitution
Given the equation for a reaction:
C4H10 + Cl2 → C4H9Cl + HCl
Which type of reaction is represented by the equation?
(1) addition
(2) substitution
(3) fermentation
(4) polymerization
Two types of organic reactions are
(1) addition and sublimation
(2) deposition and saponification
(3) decomposition and evaporation
(4) esterification and polymerization
Which type of organic reaction produces both water and carbon dioxide?
(1) addition
(2) combustion
(3) esterification
(4) fermentation
Ethanoic acid and 1-butanol can react to produce water and a compound classified as an
(1) aldehyde
(2) amide
(3) ester
(4) ether
Which equation represents an addition reaction?
(1) C3H8 + Cl2 → C3H7Cl +HCl
(2) C3H6 + Cl2 → C3H6Cl2
(3) CaCl2 + Na2CO3 → CaCO3 + 2NaCl
(4) CaCO3 → CaO + CO2
Which equation represents fermentation?
(1) C2H4 + H2O → CH3CH2OH
(2) C2H4 + HCl → CH3CH2Cl
(3) C6H12O6 → 2CH3CH2OH + 2CO2
(4) 2CH3CHO → C3H5CHO + H2O
In one method of making bread, starch is broken down into glucose. Zymase, an enzyme present in yeast, acts as a catalyst for the reaction in which the glucose reacts to produce ethanol and carbon dioxide. The carbon dioxide gas causes the bread dough to rise. The balanced equation below represents the catalyzed reaction.
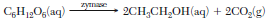
Identify the type of organic reaction represented by this equation.
Allow 1 credit. Acceptable responses include, but are not limited to:
• fermentation
One type of soap is produced when ethyl stearate and sodium hydroxide react. The soap produced by this reaction is called sodium stearate. The other product of the reaction is ethanol. This reaction is represented by the balanced equation below.
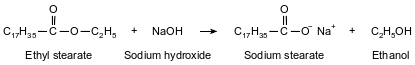
Identify the type of organic reaction used to make soap.
Allow 1 credit for saponification.
In industry, ethanol is primarily produced by two different reactions. One process involves the reaction of glucose in the presence of an enzyme that acts as a catalyst. The equation below represents this reaction.
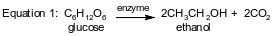
In another reaction, ethanol is produced from ethene and water. The equation below represents this reaction in which H2SO4 is a catalyst.
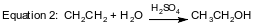
Industrial ethanol can be oxidized using a catalyst to produce ethanal. The equation representing this oxidation is shown below.

Identify the type of organic reaction represented by equation 1.
Allow 1 credit. Acceptable responses include, but are not limited to:
• fermentation
• fermenting
