Topic: Valence Electrons
Valence Electrons
Which atom in the ground state has an outer- most electron with the most energy?
(1) Cs
(2) K
(3) Li
(4) Na
The valence electron of which atom in the ground state has the greatest amount of energy?
(1) cesium
(2) lithium
(3) rubidium
(4) sodium
Compared to the energy and charge of the electrons in the first shell of a Be atom, the electrons in the second shell of this atom have
(1) less energy and the same charge
(2) less energy and a different charge
(3) more energy and the same charge
(4) more energy and a different charge
Compared to the energy of an electron in the second shell of an atom of sulfur, the energy of an electron in the
(1) first shell is lower
(2) first shell is the same
(3) third shell is lower
(4) third shell is the same
Which electron shell in an atom of calcium in the ground state has an electron with the greatest amount of energy?
(1) 1
(2) 2
(3) 3
(4) 4
Which change in electron location in an atom of calcium is accompanied by the greatest amount of energy emitted?
(1) from shell 1 to shell 2
(2) from shell 2 to shell 1
(3) from shell 1 to shell 4
(4) from shell 4 to shell 1
In the ground state, which shell of a potassium atom has an electron with the greatest amount of energy?
(1) first
(2) second
(3) third
(4) fourth
Compare the energy of an electron in the first shell of a cadmium atom to the energy of an electron in the third shell of the same atom.
Allow 1 credit. Acceptable responses include, but are not limited to:
• An electron in the first shell has less energy than an electron in the third shell.
• The third shell electron has higher energy.
• 3rd shell ⬎ 1st shell
The Bohr model of the atom was developed in the early part of the twentieth century. A diagram of the Bohr model for one atom, in the ground state, of a specific element is shown below. The nucleus of this atom contains 4 protons and 5 neutrons.
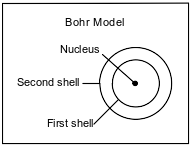
Using the Bohr model, describe the changes in electron energy and electron location when an atom changes from the ground state to an excited state.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Change in electron energy:
• Electron energy increases.. An electron absorbs energy. more energy
• Change in electron location:
• An electron moves to a higher electron shell. from the first to the second shell second to higher energy level farther from the nucleus
The table below shows data for three isotopes of the same element.
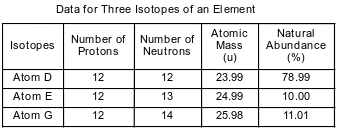
Compare the energy of an electron in the first electron shell to the energy of an electron in the second electron shell in an atom of isotope E.
Allow 1 credit. Acceptable responses include, but are not limited to:
• An electron in the first shell of an atom of isotope E has less energy than an electron in the second shell.
• In an atom of E, an electron in the 2nd energy level has more energy than an electron in the 1st energy level.
• Electrons in shell 2 have higher energies than shell 1 electrons.
• lower in shell 1
The two naturally occuring isotopes of lithium are Li-6 and Li-7. The table below shows the atomic mass and percent natural abundance for these isotopes.
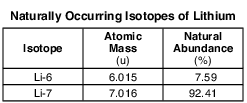
Compare the energy of an electron in the first shell of a lithium atom to the energy of an electron in the second shell of the same atom. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• second shell.
• The second shell electron has greater energy.
The four naturally occurring isotopes of sulfur are S-32, S-33, S-34, and S-36. The table below shows the atomic mass and percent natural abundance for these isotopes.
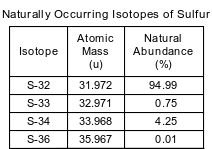
Compare the energy of an electron in the third shell of a sulfur atom to the energy of an electron in the first shell of the same atom. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• The energy of an electron in the third shell is higher than the energy of an electron in the first shell.
• The third shell electron has higher energy.
• The electron in the first shell has less.
• Note: The student response must address energy of electrons, not just shells.
The element technetium, Tc, has several isotopes. The bright-line spectrum of technetium has been observed in the spectra of some stars.
Compare the energy of an electron in the first shell of a technetium atom to the energy of an electron in the third shell of the same atom. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• An electron in the first shell of a Tc atom has less energy than an electron in the third shell.
• The third shell electron has greater energy.
• The electron in the first shell has less.
Iron has been used for thousands of years. In the air, iron corrodes. One reaction for the corrosion of iron is represented by the balanced equation below.
Equation 1: 4Fe(s) + 3O2(g) → 2Fe2O3(s)
In the presence of water, iron corrodes more quickly. This corrosion is represented by the unbalanced equation below.
Equation 2: Fe(s) + O2(g) + H2O(ℓ) → Fe(OH)2(s)
Using equation 1, describe one chemical property of iron.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Iron reacts with oxygen to form a compound.
• An iron atom can lose three electrons.
• The Fe atoms can form positive ions.
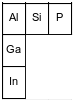
The diagram below represents three elements in Group 13 and three elements in Period 3 and their relative positions on the Periodic Table.
Some elements in the solid phase exist in different forms that vary in their physical properties. For example, at room temperature, red phosphorus has a density of 2.16 g/cm3 and white phosphorus has a density of 1.823 g/cm3.
Identify the element from the diagram that will react with chlorine to form a compound with the general formula XCl4.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Si
• silicon
• element 14
