Topic: Voltaic Cell
Voltaic Cell
What occurs at one of the electrodes in both an electrolytic cell and a voltaic cell?
(1) Oxidation occurs as electrons are gained at the cathode.
(2) Oxidation occurs as electrons are lost at the anode.
(3) Reduction occurs as electrons are gained at the anode.
(4) Reduction occurs as electrons are lost at the cathode.
In an operating voltaic cell, reduction occurs
(1) at the anode
(2) at the cathode
(3) in the salt bridge
(4) in the wire
An electrolytic cell differs from a voltaic cell because an electrolytic cell
(1) generates its own energy from a spontaneous physical reaction
(2) generates its own energy from a nonsponta- neous physical reaction
(3) requires an outside energy source for a spontaneous chemical reaction to occur
(4) requires an outside energy source for a nonspontaneous chemical reaction to occur
Which change occurs at the anode in an operating electrochemical cell?
(1) gain of protons
(2) gain of electrons
(3) loss of protons
(4) loss of electrons
Which reaction occurs at the cathode in an electrochemical cell?
(1) combustion
(2) neutralization
(3) oxidation
(4) reduction
Which term identifies the half-reaction that occurs at the anode of an operating electro- chemical cell?
(1) oxidation
(2) reduction
(3) neutralization
(4) transmutation
At which electrode does oxidation occur in a voltaic cell and in an electrolytic cell?
(1) the anode in a voltaic cell and the cathode in an electrolytic cell
(2) the cathode in a voltaic cell and the anode in an electrolytic cell
(3) the anode in both a voltaic cell and an electrolytic cell
(4) the cathode in both a voltaic cell and an electrolytic cell
Where do reduction and oxidation occur in an electrolytic cell?
(1) Both occur at the anode.
(2) Both occur at the cathode.
(3) Reduction occurs at the anode, and oxidation occurs at the cathode.
(4) Reduction occurs at the cathode, and oxidation occurs at the anode.
Which reaction occurs at the anode of an electrochemical cell?
(1) oxidation
(2) reduction
(3) neutralization
(4) transmutation
Which reaction occurs at the anode in an electrochemical cell?
(1) oxidation
(2) reduction
(3) combustion
(4) substitution
The diagram below represents an operating electrolytic cell used to plate silver onto a nickel key. As the cell operates, oxidation occurs at the silver electrode and the mass of the silver electrode decreases.
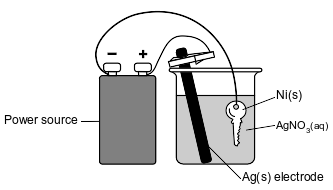
Identify the cathode in the cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Ni(s) key
• key
• nickel
A student constructs an electrochemical cell. A diagram of the operating cell and the unbalanced ionic equation representing the reaction occurring in the cell are shown below.
The blue color of the solution in the copper half-cell indicates the presence of Cu2+ ions. The student observes that the blue color becomes less intense as the cell operates.
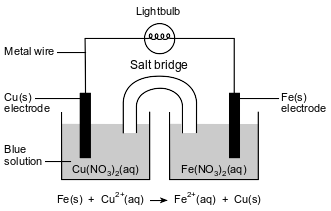
Identify the type of electrochemical cell represented by the diagram.
Allow 1 credit. Acceptable responses include, but are not limited to:
• voltaic cell
• voltaic
• Galvanic
An operating voltaic cell has magnesium and silver electrodes. The cell and the ionic equation representing the reaction that occurs in the cell are shown below.
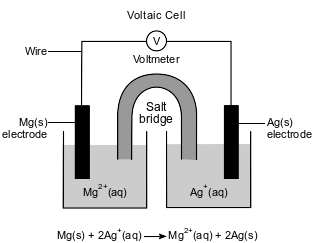
Explain, in terms of electrical energy, how electrolysis reactions differ from voltaic cell reactions.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Electrical energy is required for electrolytic reactions, while voltaic cell reactions produce electricity.
• Voltaic cells produce electrical energy, and electrolytic cells use electrical energy.
• Electrolysis reactions require an external source of electricity.
Electroplating is an electrolytic process that can be used to coat metal objects with a less reactive metal. The diagram below shows an electroplating cell that includes a power source connected to a copper rod and a bracelet made from a different metal. The rod and bracelet are in an aqueous copper(II) sulfate solution.
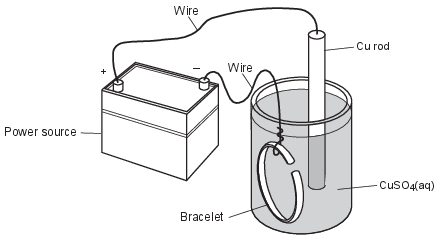
Identify the electrode that attracts the Cu2+ ions as the cell operates. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• The Cu2+ ions are attracted to the bracelet.
• bracelet
• negative electrode
• cathode
A student made a copper bracelet by hammering a small copper bar into the desired shape. The bracelet has a mass of 30.1 grams and was at a temperature of 21°C in the classroom. After the student wore the bracelet, the bracelet reached a temperature of 33°C. Later, the student removed the bracelet and placed it on a desk at home, where it cooled from 33°C to 19°C. The specific heat capacity of copper is 0.385 J/g•K.
Explain, in terms of chemical activity, why copper is a better choice than iron to make the bracelet.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Copper is less chemically active than iron, so copper is less likely to react with substances in the air or on the skin.
• Iron is more active.
• Fe oxidizes more easily.
