Topic: Wave Mechanical Model
Wave Mechanical Model
Which atom in the ground state has an outer- most electron with the most energy?
(1) Cs
(2) K
(3) Li
(4) Na
In the wave-mechanical model of the atom, an orbital is the most probable location of
(1) a proton
(2) a positron
(3) a neutron
(4) an electron
What is the total number of valence electrons in a germanium atom in the ground state?
(1) 22
(2) 2
(3) 32
(4) 4
An orbital is defined as a region of the most probable location of
(1) an electron
(2) a neutron
(3) a nucleus
(4) a proton
According to the wave-mechanical model, an orbital is defined as the
(1) circular path for electrons
(2) circular path for neutrons
(3) most probable location of electrons
(4) most probable location of neutrons
In the ground state, an atom of each of the elements in Group 2 has a different
(1) oxidation state
(2) first ionization energy
(3) number of valence electrons
(4) number of electrons in the first shell
Which term identifies the most probable location of an electron in the wave-mechanical model of the atom?
(1) anode
(2) orbital
(3) nucleus
(4) cathode
Which element has atoms in the ground state with the greatest number of valence electrons?
(1) tin
(2) sulfur
(3) arsenic
(4) fluorine
In the ground state, all atoms of Group 15 elements have the same number of
(1) valence electrons
(2) electron shells
(3) neutrons
(4) protons
In the ground state, valence electrons of a krypton atom are found in
(1) the first shell
(2) the outermost shell
(3) both the nucleus and the first shell
(4) both the first shell and the outermost shell
There are six elements in Group 14 on the Periodic Table. One of these elements has the symbol Uuq, which is a temporary, systematic symbol. This element is now known as flerovium.
State the expected number of valence electrons in an atom of the element flerovium in the ground state.
Allow 1 credit. Acceptable responses include, but are not limited to:
• 4
• four
• 4e−
• four valence electrons
Rubidium and iodine have different chemical and physical properties. Some of these properties are shown in the table below.
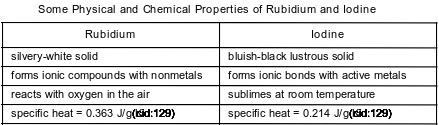
Compare the atomic radius of an atom of iodine to the atomic radius of an atom of rubidium when both atoms are in the ground state.
Allow 1 credit. Acceptable responses include, but are not limited to:
• In the ground state, the atomic radius of an iodine atom is smaller than the atomic radius of a rubidium atom.
• The Rb atom is larger than the I atom.
• The Rb atomic radius is 215 pm, but the I atomic radius is only 136 pm.
Periodic trends are observed in the properties of the elements in Period 3 on the Periodic Table. These elements vary in physical properties, such as phase, and in chemical properties, such as their ability to lose or gain electrons during a chemical reaction.
Identify the element in Period 3 that requires the least amount of energy to remove the most loosely held electrons from a mole of gaseous atoms of the element in the ground state.
Allow 1 credit for Na or sodium.
The Bohr model of the atom was developed in the early part of the twentieth century. A diagram of the Bohr model for one atom, in the ground state, of a specific element is shown below. The nucleus of this atom contains 4 protons and 5 neutrons.
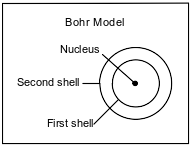
State the number of electrons in each shell in this atom in the ground state.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Number of electrons in first shell: 2 or 2e−
• Number of electrons in second shell: 2 or 2e−
The table below shows data for three isotopes of the same element.
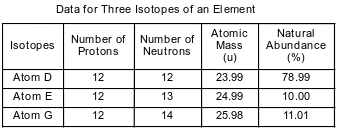
State the number of valence electrons in an atom of isotope D in the ground state.
Allow 1 credit for 2 or two.
