Topic: Radioactive Isotopes Dating
Radioactive Isotopes Dating
The graph below shows the rate of decay of a radioactive isotope through two half-lives. Each box shows the ratio of atoms of the radioactive isotope to atoms of the disintegration product. The box at two half-lives has been left blank.
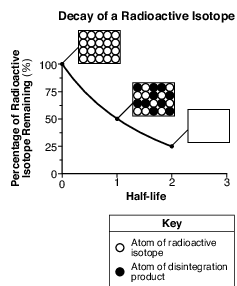
Which box best represents the ratio of these atoms at two half-lives?
(1) 
(2) 
(3) 
(4) 
Due to radioactive decay, an igneous rock sample now contains one-fourth of the amount of potassium-40 that it originally contained. The age, in years, of this rock sample is approximately
(1) 0.7 109 y
(2) 1.3 109 y
(3) 2.6 109 y ×× ××
(4) 5.2 109 y
The graph below shows the decay of a radioactive material over time.
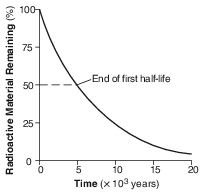
How long does it take for this radioactive material to decay through 2 half-lives?
(1) 1 × 103 years
(2) 5 × 103 years
(3) 10 × 103 years
(4) 40 × 103 years
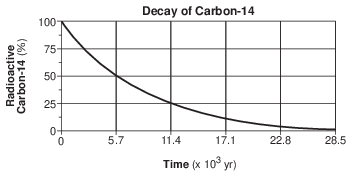
A bar graph of the radioactive decay of carbon-14 is shown below.
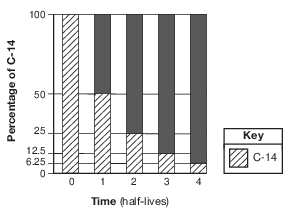
The solid black sections of the bars on the graph represent the percentages of
(1) carbon-14 from the original sample that has not decayed
(2) uranium-238 from the original sample that has not decayed
(3) nitrogen-14 decay product resulting from the radioactive decay
(4) lead-206 decay product resulting from the radioactive decay
How old is a bone that has 12.5% of the original amount of radioactive carbon-14 remaining?
(1) 5,700 years
(2) 11,400 years
(3) 17,100 years
(4) 22,800 years
The graph below shows the radioactive decay of rubidium-87.

What percentage of rubidium-87 atoms will be left after four half-lives?
(1) 25.0%
(2) 12.5%
(3) 6.25%
(4) 3.125%
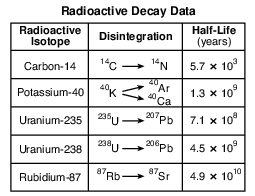
Which radioactive isotope takes the greatest amount of time to disintegrate?
(1) potassium-40
(2) uranium-235
(3) uranium-238
(4) rubidium-87
Which radioactive isotope is most often used when determining the age of fossil bones found in sediments deposited during the Holocene Epoch?
(1) carbon-14
(2) potassium-40
(3) uranium-238
(4) rubidium-87
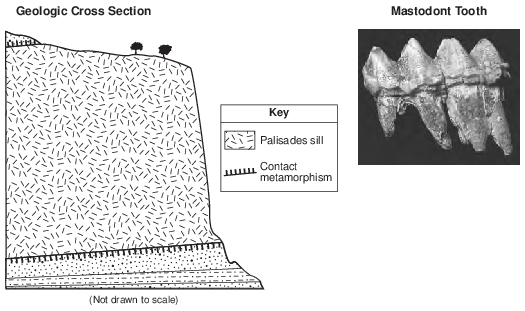
Potassium-40 is useful for radioactive dating of the Palisades sill because the half-life of potassium-40
(1) decreased as the amounts of 40Ar and 40Ca in the sill increased
(2) remained constant during the radioactive decay process
(3) increased as pressure from the overlying sedimentary rock increased
(4) was shortened by the high temperature of the magma that formed the sill
Identify the decay product when carbon-14 undergoes radioactive disintegration. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• — 14N
• — nitrogen-14/N-14
• — nitrogen/N
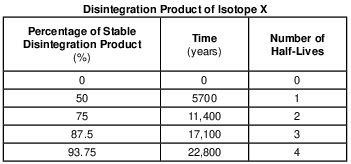
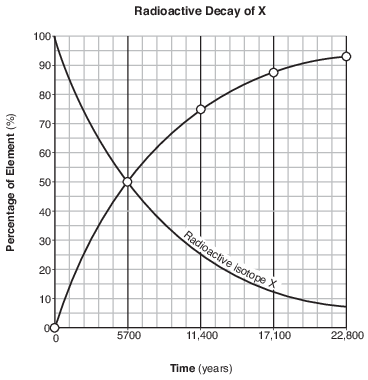
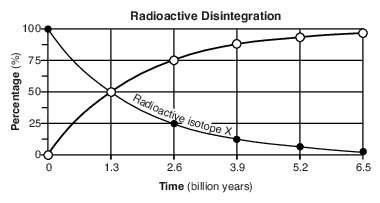
On the graph in the image provided, plot the percentage of stable disintegration product for each of the times shown on the data table and connect all five plots with a line. [1]
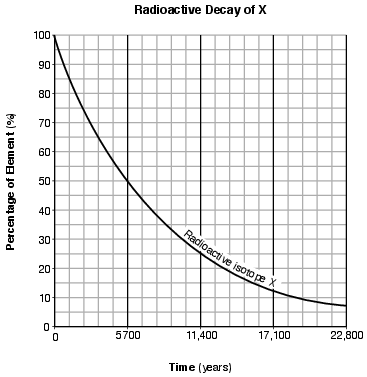
Allow 1 credit if the centers of all five plots are within or touch the circles shown and are correctly connected with a line that passes within or touches each circle.
• Note: Allow credit if the student-drawn line does not pass through the student plots, but is still
• within or touches the circles.
• It is recommended that an overlay of the same scale as the student answer booklet be used to ensure reliability in rating.
• Example of a 1-credit response:
• 
On the diagram in the image provided, shade in the correct number of carbon-14 boxes to represent the additional disintegration product after the second half-life. [1]
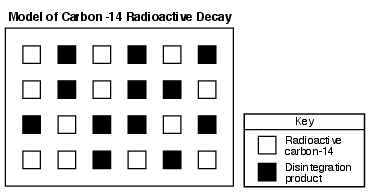
Allow 1 credit for shading in any six additional carbon-14 boxes (leaving only six boxes unshaded).
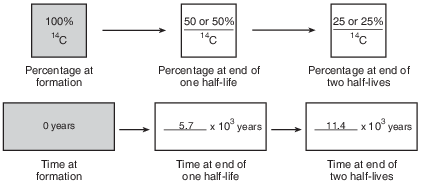
Complete the flow chart in the image provided by filling in the boxes to indicate the percentage of carbon-14 remaining and the time that has passed at the end of each half-life. [1]
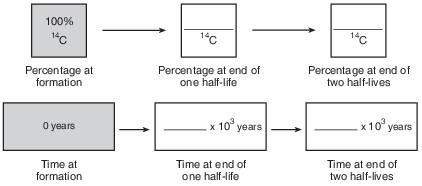
Allow 1 credit if all of the percentages and ages are correct, as shown below.
• 
•
• Note: Allow credit if the student shades the second box 50% and the third box 25% in the first
• row of boxes.

On the grid in the image provided, construct a line graph by plotting the percentages of disintegration product Z forming over 6.5 billion years. Connect all six plots with a line. The percentages of radioactive isotope X have already been plotted. [1]
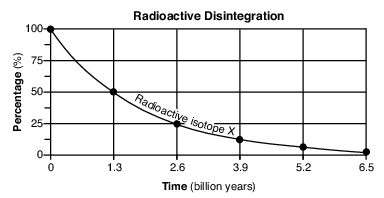
Allow 1 credit if the centers of all six plots are within or touch the circles shown and are correctly connected with a line that passes within or touches each circle.
• Note: Allow credit if the student-drawn line does not pass through the student plots, but is still
• within or touches the circles. It is recommended that an overlay of the same scale as the student answer booklet be used to ensure reliability in rating.
• 
X The geologic time period when each sedimentary rock layer formed is shown. The symbols ( , , , , and ) represent fossils of different types of organisms present in the rock layers.
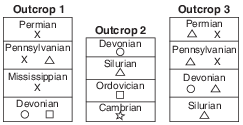
Explain why the geologic age of these rock layers could not be accurately dated using carbon-14. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• — Carbon-14 has a short half-life.
• — These rock layers are too old to contain measurable carbon-14.
• — Carbon-14 is used to date recent remains.
• — No organic material remains in the rock.
